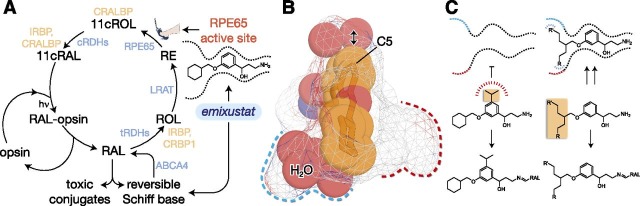
Fig. 1.
Strategies for selective modulation of the retinoid cycle. (A) Emixustat modulates visual cycle function both by inhibition of 11-cis-retinol production through direct binding to the RPE65 active-site pocket, as well as by temporary sequestration of retinaldehyde through the formation of a Schiff base conjugate. Both modes of action can theoretically suppress the formation of toxic conjugates between retinaldehyde and cellular constituents that trigger retinal degeneration. Enzymes and retinoid-binding proteins of the visual cycle pathway are shown in blue- and peach-colored text, respectively. 11cRAL, 11-cis-retinal; 11cROL, 11-cis-retinol; CRALBP, cellular retinaldehyde-binding protein; CRBP1, cellular retinol-binding protein 1; tRDH, 11-cis-retinol dehydrogenase; IRBP, interphotoreceptor retinoid-binding protein; LRAT, lecithin:retinol acyltransferase; RAL, all-trans-retinaldehyde; RE, all-trans-retinyl ester; ROL, all-trans-retinol; RAL, all-trans-retinol dehydrogenase. (B) Structure of the emixustat-binding region of the RPE65 active-site cavity. Ligands and water molecules are shown as full-scale van der Waals spheres with carbon, nitrogen, and oxygen atoms colored orange, blue, and red, respectively, whereas the binding pocket is shown as a Connolly surface. The arrow emphasizes the relatively snug fit of the phenyl ring in the vicinity of carbon 5 (C5) to the active-site pocket. The water-filled polar (left) and apolar (right) binding pockets are delineated with blue and red dashed lines, respectively, on opposite sides of the emixustat cyclohexyl ring. This figure was generated using coordinates deposited under the Protein Data Bank accession code 4RSC in PyMOL (Schrödinger, LLC). (C) Structure-guided strategies to promote or block either of the two modes of visual cycle modulation exerted by emixustat. In the left panel, addition of a bulky hydrophobic substituent to the central phenyl ring should sterically prevent binding of the emixustat derivative to the RPE65 active-site pocket while simultaneously preserving RAL sequestration activity. In the right panel, design of inhibitors that occupy unused pockets within the RPE65 active-site cavity could improve potency and thus enable more selective RPE65 inhibition at lower drug concentrations.