Figure 2. WNT and SHH signaling pathways.
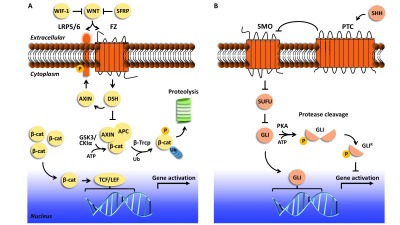
( A) The WNT signaling pathway is mediated by the receptor Frizzled (FZ) and single-pass low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6). In the pathway’s “off” state (in the event of low, no, or WNT ligand function inhibited by WNT inhibitor factor 1 [WIF-1] or secreted frizzle-related protein [SFRP]), β-catenin (β-cat) is targeted for phosphorylation by glycogen synthase kinase 3 (GSK3) and casein kinase I alpha (CKIα), aided by proteins AXIN and adenomatous polyposis coli (APC). β-catenin is then ubiquitinated and targeted for proteolysis by the proteasome. In the pathway’s “on” state, WNT ligand is recognized by FZ and LRP5/6, and LRP5/6 is phosphorylated. The WNT-FZ-LRP5/6 trimeric complex triggers the recognition of Dishevelled (DSH) and AXIN. β-catenin is not phosphorylated, translocates to the nucleus, and functions as a transcriptional coactivator to activate TCF/LEF family transcription factors. Prominent drug targets that aim to regulate WNT-responsive gene expression 22 include those that target (1) extracellular events, such as recognition of WNT by FZ and/or LRP5/6 (vantictumab and ipafricept), (2) cytoplasmic events, such as inhibition of DSH or stabilization of the AXIN/APC interaction (IWR-1; XAV939; 3289-8625; FJ9; NSC 668036; JW74), and (3) transcriptional activation, such as perturbing β-catenin function (PFK115-584; CGP049090; iCRT-3, -5, and -14; PRI-724). There are still other drugs that target events involved in WNT secretion to the extracellular space as well as other enzymes that regulate the pathway, but they are not shown in this schematic. Ub, ubiquitin. ( B) The sonic hedgehog (SHH) pathway is mediated by the receptors Smoothened (SMO) and Patched (PTC). In the pathway’s “off” state (in the event of low or no SHH ligand), SMO transport from intracellular vesicles to the membrane and its activity at the membrane are inhibited, in part by PTC. Members of transcription factor family GLI are inhibited by suppressor of fused (SUFU). Protein kinase A (PKA) phosphorylates the GLI transcription factors, which undergo proteasomal cleavage to yield a functional repressor form (GLI R). GLI R translocates to the nucleus and inhibits target gene expression. In the pathway’s “on” state, SHH binds to and inhibits PTC and SUFU is inhibited. SMO levels at the membrane increase, leading to activation of GLI transcription factors, which translocate to the nucleus to activate SHH-responsive genes. Prominent drug targets that aim to regulate SHH-responsive gene expression 13 include those that target (1) extracellular events, such as SMO function, including by inhibition of SHH (purmorphamine, cyclopamine, vismodegib 12; sonidegib or Odomzo®, jervine; saridegib, CUR 61414, BMS-833923, glasdegib, PF-5274857, TAK-441, Taladegib, and SANT-1) and its binding to PTC (5E1, a monoclonal antibody), and (2) transcription activation, such as regulating GLI transcriptional activation (GANT61 and arsenic trioxide).
