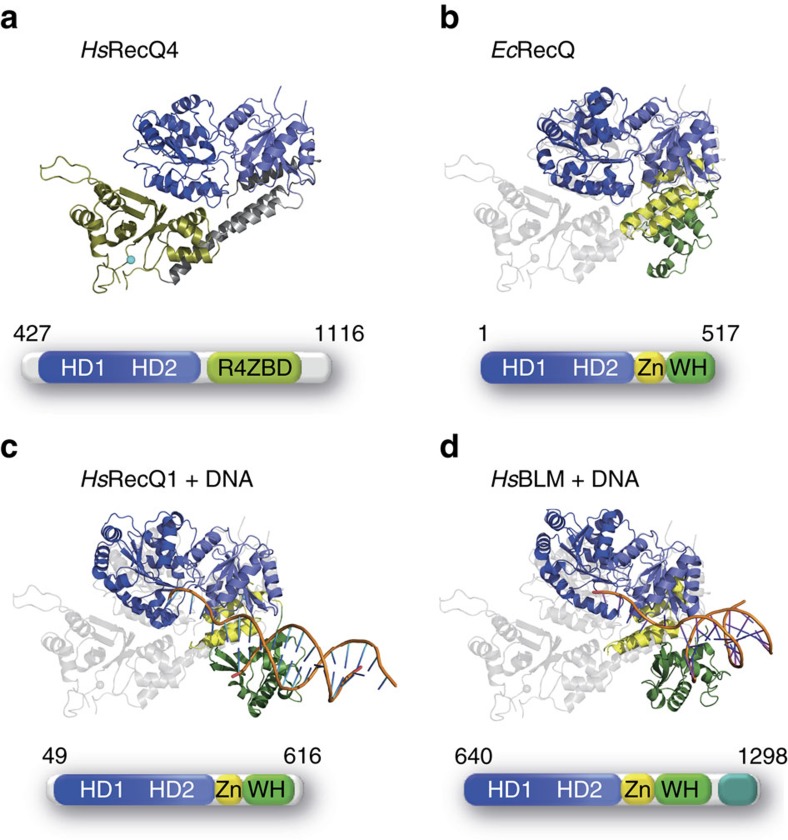
Figure 3. Comparison of RecQ4 with other RecQ structures.
Structural alignment of the HsRecQ4 structure (a) with the structures of EcRecQ (PDB 1oyw) (b), HsRecQ1 (PDB 2wwy) (c) and HsBLM (PDB 4o3m) (d). Alignments were created in pymol. All structures were superimposed utilizing the isolated HD2 of HsRecQ4. HsRecQ4 is depicted in gray within each alignment for convenient comparison. Constructs used for crystallization of each RecQ variant are illustrated below the alignment, with the colour code and abbreviations as in Fig. 1a. The alignments demonstrate that the R4ZBD of RecQ4 assumes a unique structural fold, which does not resemble the typical RQC domain of other RecQ helicases. Furthermore, the R4ZBD acquires a unique position within the RecQ4 structure, adjacent to HD1, while all RQC domains are consistently located adjacent to the HD2. The two DNA-complex structures (c,d) depict a RecQ-conserved mode of DNA binding, with the ssDNA bound across the ATPase domain and the dsDNA portion bound via the WH domain. (Note: The HsBLM structure (4o3m) also features the HRDC domain, which was omitted for the purpose of a uniform comparison).