An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.
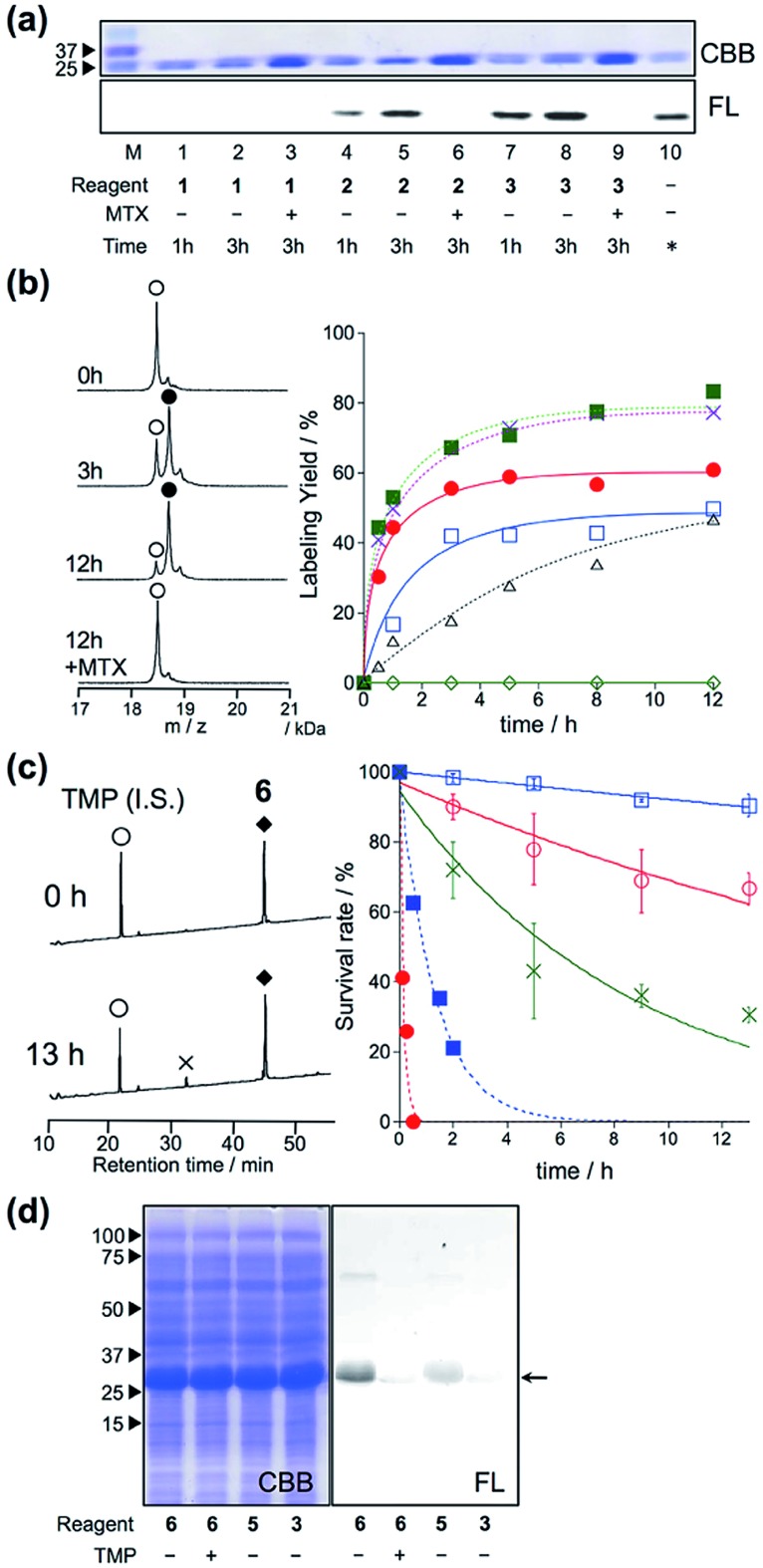
 denotes native eDHFR and
denotes native eDHFR and  denotes labeled eDHFR. (right) Time profiles of eDHFR (10 μM) labeled with 1 (
denotes labeled eDHFR. (right) Time profiles of eDHFR (10 μM) labeled with 1 ( ), 2 (
), 2 ( ), 3 (
), 3 ( ), 5 (
), 5 ( ), 6 (
), 6 ( ) and 7 (LDAI reagent,
) and 7 (LDAI reagent,  ) in buffer at 37 °C. The labeling reaction was monitored by MALDI-TOF MS analyses. (c) (left) HPLC charts of labeling reagent 6 (
) in buffer at 37 °C. The labeling reaction was monitored by MALDI-TOF MS analyses. (c) (left) HPLC charts of labeling reagent 6 ( ) in buffer solution at 37 °C for 0 h (top) or 13 h (bottom) of incubation.
) in buffer solution at 37 °C for 0 h (top) or 13 h (bottom) of incubation.  denotes decomposed reagent 6.
denotes decomposed reagent 6.  denotes the internal standard. (right) HPLC analyses of the stability of labeling reagents 3, 5 and 6 in the absence (
denotes the internal standard. (right) HPLC analyses of the stability of labeling reagents 3, 5 and 6 in the absence ( ; 3,
; 3,  ; 5,
; 5,  ; 6) or presence (
; 6) or presence ( ; 3,
; 3,  ; 6) of porcine liver esterase (100 nM) in buffer solution at 37 °C. (d) SDS-PAGE analysis of the labeling of Trx–His6–eDHFR with 6 (10 μM) in E. coli lysates.
; 6) of porcine liver esterase (100 nM) in buffer solution at 37 °C. (d) SDS-PAGE analysis of the labeling of Trx–His6–eDHFR with 6 (10 μM) in E. coli lysates.