Figure 4. Expansion of human cells on decellularized plant stems.
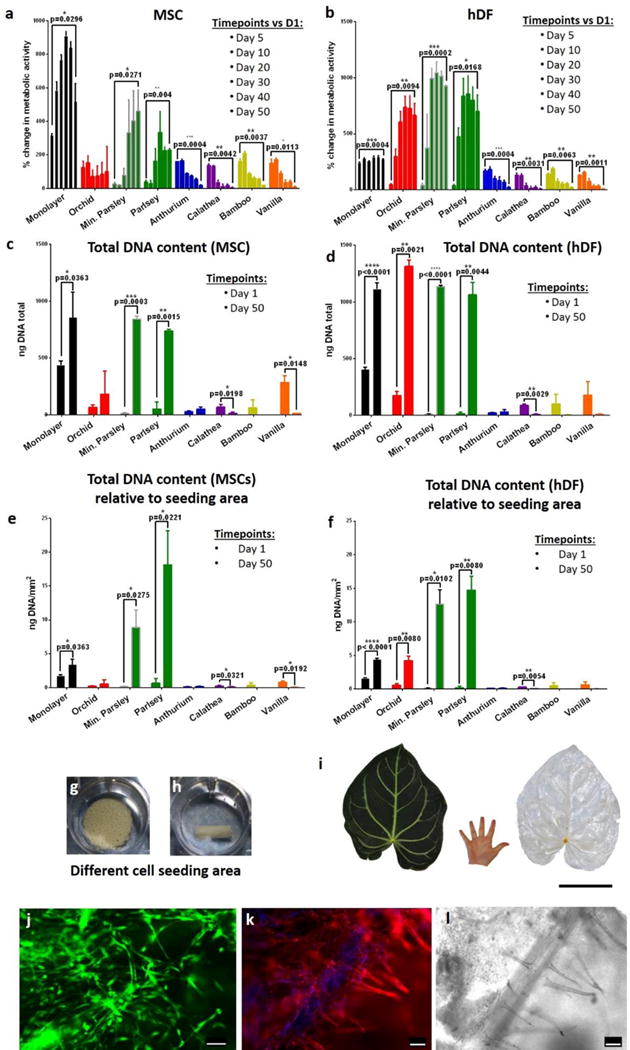
a–b, The metabolic activity of cells was measured using the CellTiter-Blue® assay. MSCs (a) show a steady increase in metabolic activity only on monolayer and on parsley stems, and they decrease in other plant stems. Similar behavior was observed also in hDF (b), however, in this case there was a significant increase in metabolic activity also in orchid’s pseudobulb stems. n=3, p<0.05 paired student’s t-test. c–d, Quantification of total DNA content assessed using the PicoGreen® assay and compared between day 1 and day 50 of culture, n=3, p<0.05 paired student’s t-test. e–f, Total DNA content (ng/mm2) was normalized by seeding area; n=3, p<0.05 paired student’s t-test. g–h, Orchid’s pseudobulb and a mineralized parsley stem respectively in ultralow attachment polystyrene wells. They clearly have different volumes and offer different cell seeding areas. i, Tropical Anthurium magnificum leaf before and after decellularization. The leaves of Anthurium magnificum are on average 30 cm wide and 40 cm long, in the images their size is directly compared to that of a human hand. Scalebar 15cm. j, A decellularized Anthurium magnificum leaf was cut using 8 mm biopsy punch and was used as scaffold for culture of HUVEC cells. After 5 days of culture, live cells were stained using calcein (green). K–l, Rhodamine-phalloidin staining (k) of actin filaments (red) and DAPI staining of nuclei (blue) and relative brightfield image (l) of HUVEC cells cultured on the decellularized Anthurium magnificum leaf for 5 days. The brightfield image (l) dislays the presence of vascular structures and cells appear to register the shape of the vessels (k). From a single leaf of Anthurium magnificum it was possible to obtain numerous pre-vascularized scaffolds. Scalebars 100 μm.
