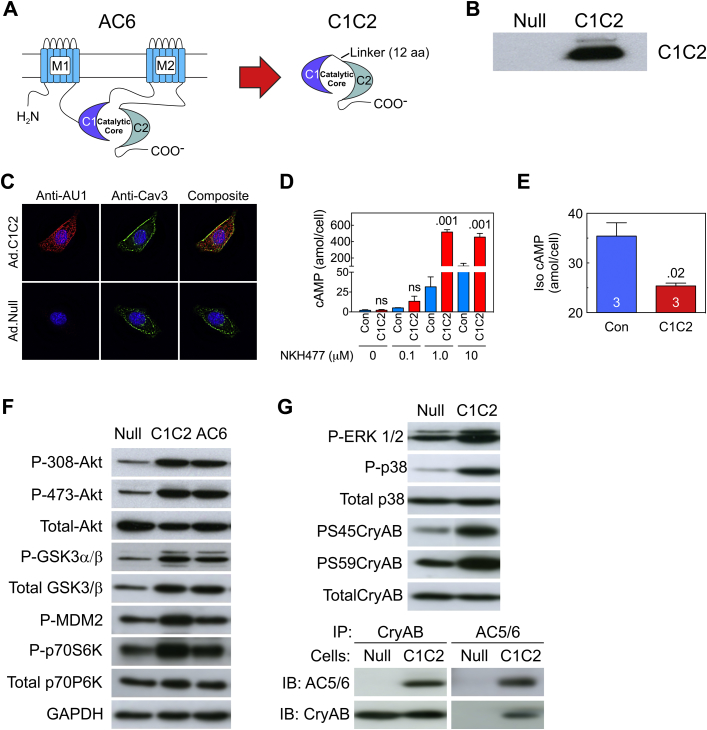
Figure 1.
C1C2 Design, Expression, Cellular Distribution, Activity, and Intracellular Signaling
(A) C1C2 construct that forms the catalytic core. M1 and M2, transmembrane domains of AC6; C1 and C2, cytoplasmic domains of AC6; Linker, 12 amino acids. (B) C1C2 protein was detected in immunoblotting using an anti-AU1 tag antibody in NRCM after gene transfer with Ad5.C1C2 (200 vp/cell). (C) Double-immunofluorescence staining of C1C2 protein in NRCM using anti-AU1 antibody (red); anti-caveolin 3 (Cav-3) antibody (green). Yellow indicates co-localization of C1C2 with caveolin. (D, E) NRCM underwent Ad5.C1C2 gene transfer and the amount of cAMP production in response to NKH477, a forskolin analog (D) or 10 μM Iso (10 min) (E). Cardiac myocytes expressing C1C2 showed increased catalytic activity in a dose-dependent manner after stimulation with NKH477 (D) (activates AC) but reduced cAMP activity in response to βAR (Iso) stimulation (E). Results were confirmed in 3 separate experiments. A representative experiment is shown with triplicate samples. Bars denote mean ± SE; p values are from Student′s t-test (unpaired, 2-tailed). (F) Immunodetection of molecules in the Akt signaling pathway indicate that C1C2 and AC6 expression were associated with similar increases in phosphorylation of Akt, GSK3α/β, MDM2, and p70S6k, suggesting that the effect does not require AC6-mediated cAMP production. (G) C1C2 expression was associated with phosphorylation of ERK1/2, p38 MAPK, and αB-crystallin (CryAB, upper). Interaction of CryAB and C1C2 detected by co-immunoprecipitation and immunoblotting (lower). In A to E, Con denotes transgene negative mice. Iso = isoproterenol; NRCM = neonatal rat cardiac myocytes.