Figure 1.
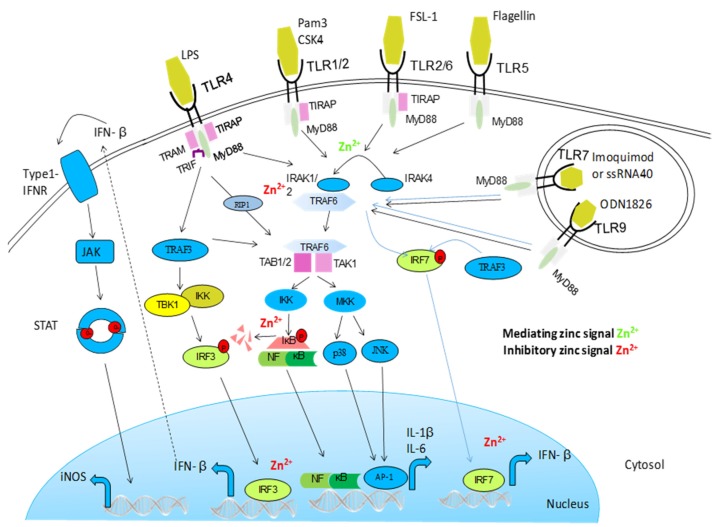
Activation of Toll-like receptor (TLR) signaling pathways is mediated by a complex array of proteins. When TLRs bind ligands, dimerization of the ectodomain of TLRs is induced, bringing their cytoplasmic Toll/IL-1R domains together, resulting in the recruitment of intracellular adapter proteins and initiation of downstream signaling events. Following this, the Toll–IL-1-resistence (TIR) domains of TLRs engage TIR domain-containing adaptor proteins (either myeloid differentiation primary-response protein 88 (MYD88) or TIR domain-containing adaptor protein inducing IFNβ (TRIF) and TRIF-related adaptor molecule (TRAM)). This in turn stimulates downstream signaling pathways that involve interactions between IL-1R-associated kinases (IRAKs) and the adaptor molecules TNF receptor-associated factors (TRAFs), and that lead to the activation of the mitogen-activated protein kinases (MAPKs) JUN N-terminal kinase (JNK) and p38, and to the activation of transcription factors. Two groups of transcription factors are activated downstream of TLR signaling; nuclear factor-κB (NF-κB) and interferon-regulatory factors (IRFs). This TLR signaling pathway leads to the induction of proinflammatory cytokines. Zinc influences this pathway on multiple levels. It augments MyD88-dependent signaling whereas inhibiting TRIF-mediated activation of IRF3/7. It has been shown that zinc inhibits IRAK thereby inhibiting further signal transductions. In vitro, zinc also directly impaired LPS-induced IKK activity [45].