Figure 1.
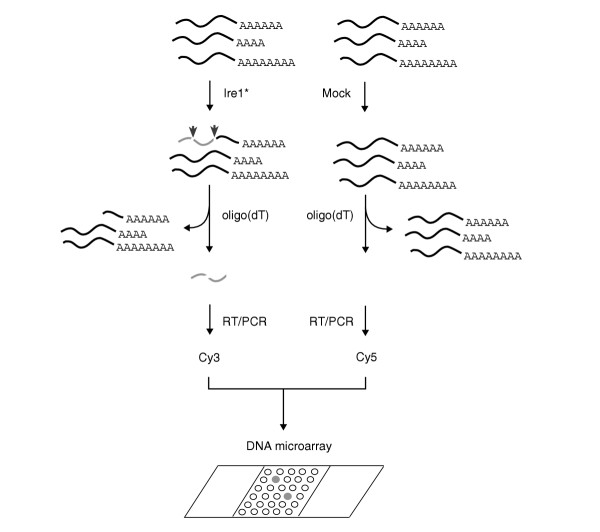
Schematic of screen for RNA substrates of Ire1p endoribonuclease using an in vitro nuclease reaction. Recombinant Ire1* expressed and purified from E. coli is incubated with poly(A)+ RNA isolated from wild-type S. cerevisiae to cleave endogenous HAC1 mRNA and other potential RNA substrates of Ire1p. Cleaved RNA (lacking the poly(A) tail) is separated from uncleaved RNA as the unbound, poly(A)- fraction from an oligo(dT) column and used to prepare fluorescent probe by reverse transcription followed by PCR amplification in the presence of Cy3-dTTP. A second control probe, using poly(A)- RNA from mock nuclease reactions (identical reactions except for the lack of Ire1*) is prepared similarly, except that PCR amplification was carried out in the presence of Cy5-dTTP. Equal amounts of these probes are mixed and used to probe the yeast DNA microarray. Because RNA fragments generated by Ire1* cleavage are represented only in the Cy3 probe, microarray spots hybridizing to cleaved fragments should appear green, whereas microarray spots hybridizing to molecules common to both probes should appear yellow upon superimposition of green (Cy3) and red (Cy5) channels.
