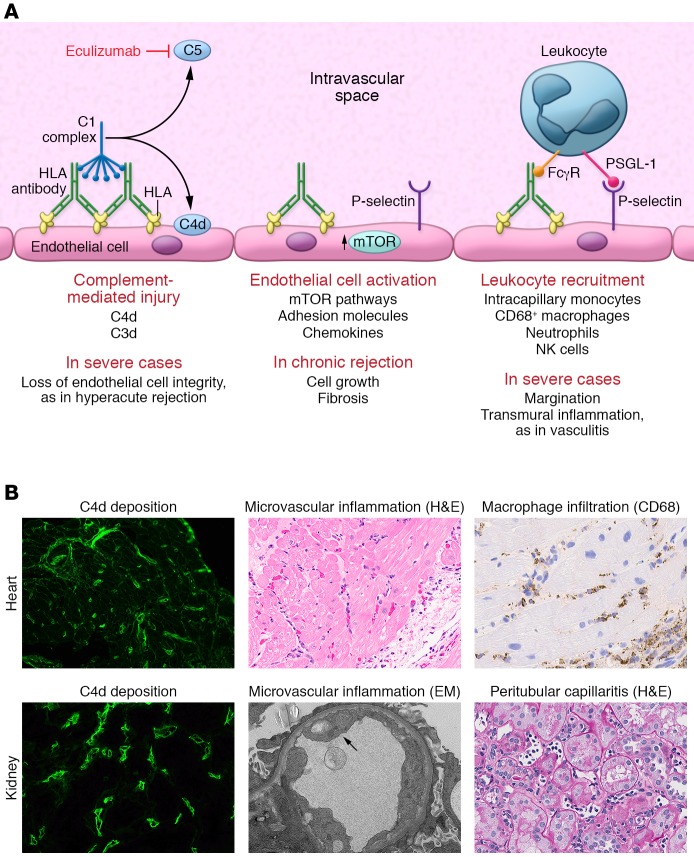
Figure 2. The known mechanisms of HLA antibody–mediated allograft injury and their therapeutic targets.
(A) HLA antibody binding to donor endothelial cells can trigger activation of the classical complement cascade. First, the complement C1 complex, which includes C1q, C1r, and C1s, binds to the IgG heavy chain Fc region. The C1 complex next sequentially activates serum complement proteins, catalyzing the generation of immunologically active split products. Activation of complement C5 protein occurs at the terminal stage of the signaling pathway, generating the highly potent anaphylatoxin C5a and initiating assembly of the membrane attack complex (MAC). In the process, the split product C4d becomes covalently bound to the endothelial cell surface and can be detected in biopsies of allografts undergoing rejection through immunofluorescent or immunohistochemical staining. Eculizumab is an mAb that prevents C5 cleavage. HLA binding also activates intracellular signaling within the donor endothelium, mainly via the mTOR signaling axis, and upregulation of the adhesion molecule P-selectin. P-selectin, in conjunction with the interaction between immune cell Fcγ receptors (FcγRs), enhances leukocyte-endothelial adhesion. PSGL-1, P-selectin glycoprotein 1. (B) Representative micrographs illustrating the three main histological features of AMR in heart (top panels) and kidney (bottom panels) allografts. Deposition of C4d within the microvasculature is visualized by immunofluorescence in green. H&E staining demonstrates increased capillary endothelial cell size and numerous leukocytes in the intravascular space. Immunohistochemical staining for CD68 highlights intracapillary macrophages (original magnification, ×400). EM, electron micrograph.