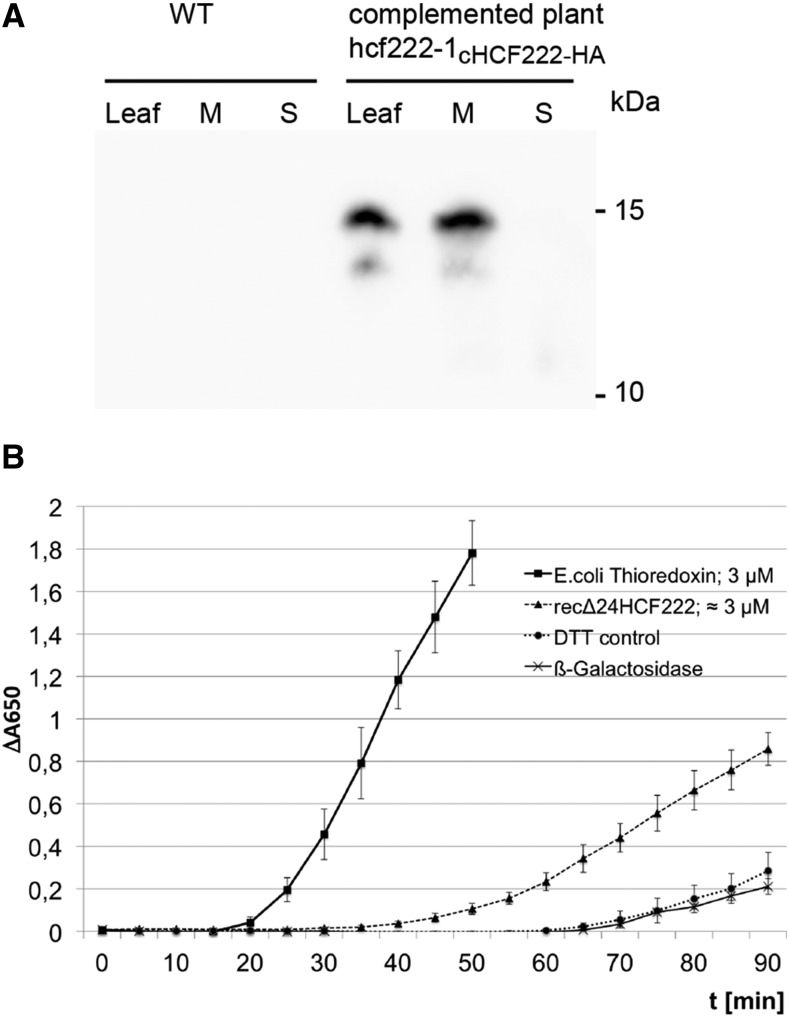
Figure 8.
Characterization of HCF222. A, Immunodetection of HCF222-HA in leaf extracts. After SDS-PAGE, the protein pattern of whole-leaf extracts (Leaf) and whole-leaf membrane (M) and soluble fraction (S) from wild-type (WT) and complemented plants was transferred to nitrocellulose, and the fusion protein was detected with anti-HA antibody. B, Measurement of disulfide reductase activity of recombinant HCF222-6xHis by the reductive insulin assay. The reduction of disulfide bonds in insulin was monitored by an increase in A650, which results from insulin precipitation. The concentration of recombinant HCF222-6xHis was estimated after SDS-PAGE and Coomassie Blue staining in comparison with a dilution series of lysozyme. DTT without enzyme served as a control. In addition, recombinant β-galactosidase-6xHis from similarly purified E. coli extracts served as a negative control to estimate contaminants from E. coli. Values represent means of three independent experiments, each with three replicates; β-galactosidase activity is shown as means of three replicates from one purification. Means with sd are depicted.