Abstract
Obesity relatively late in adulthood has been consistently associated with increased risk of primary liver cancer. However, little is known about the role of early adult adiposity and evolution of adiposity across adulthood in hepatocarcinogenesis. We examined adult body mass index (BMI; kg/m2) in relation to hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) in a large prospective cohort. Weight at ages 18, 35, 50 and at study baseline was retrospectively reported by 303,620 participants. BMI trajectories were identified using latent class trajectory modeling. Incidence of HCC and ICC was determined through 2011. Cox proportional hazards modeling was used to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). A total of 372 HCC cases and 104 ICC cases occurred during follow-up. Being obese (BMI ≥ 30) at ages 18, 35, 50 and at baseline (mean age 62.3 years, range 50.3–71.5 years) was associated with an 86%–119% elevated risk of HCC. BMI trajectories that resulted in obesity were associated with ~80% higher HCC incidence. BMI at age 18, per 5 kg/m2, was associated with a 34% higher risk of ICC, but the association attenuated for BMI at older ages. In conclusion, our findings suggest that maintaining a healthy BMI throughout the lifetime may reduce liver cancer risk. Future studies with longitudinally collected weight information are warranted to further elucidate the role of life-course adiposity in liver cancer development.
Keywords: body mass index, liver cancer, trajectory modeling, life course epidemiology
INTRODUCTION
Liver cancer, of which hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are the most common types, is the sixth most commonly occurring cancer in the world and the second leading cause of cancer-related mortality.1 The incidence and mortality of liver cancer have been rising sharply in the United States (U.S.) and other low-risk areas over the past few decades.2–4 The role of obesity in liver cancer development has been examined in many studies.5 According to a meta-analysis of 26 prospective studies, obesity is associated with a roughly two-fold increased risk.6 Although it is not as strongly associated with liver cancer as other factors such as hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, obesity is becoming an increasingly important risk factor for liver cancer because of its growing prevalence worldwide.7 For example, in the U.S., approximately 36% of adults in 2011–2014 were obese (body mass index [BMI] ≥ 30 kg/m2),8 and obesity combined with diabetes has the greatest population attributable fraction for HCC among older adults (≥ 65 years).9
In most studies of liver cancer, obesity has been self-reported at study baseline, relatively late in adulthood. Only two prior studies examined early life BMI, and found that childhood10 or early adulthood11 obesity was associated with higher risk of liver cancer in adults. However, it remains unclear whether these associations were independent of obesity later in life. Due to the correlation of early and late life adiposity,12 simply adjusting for late-life adiposity in the model could be problematic.13 One study reported that weight gain during adulthood is associated with a higher risk of HCC; however, recalled weight was only available at age 20 (or age 25 in one study site).14 While a trajectory-based approach has been used to examine adiposity across adulthood and risk of several types of cancer,15 this method has not been used previously to study liver cancer. In addition, most prior studies of obesity and liver cancer either evaluated primary liver cancer overall, or focused on HCC, whereas few studies also examined ICC.
Herein, the associations of age-specific BMI and BMI trajectories across adulthood were examined with respect to the incidence of HCC and ICC.
MATERIALS AND METHODS
Study Population
The NIH–AARP Diet and Health study is a large prospective cohort study of persons in the U.S.16 At baseline (1995–1996), 3.5 million AARP members aged 50 to 71 years who resided in six states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia; and Detroit, Michigan) were invited to complete a questionnaire on demographic, diet, and lifestyle characteristics. Questionnaires were completed satisfactorily by 566,398 participants. Six months after completing the baseline questionnaire, a second questionnaire was mailed to living participants who did not have self-reported colon, breast, or prostate cancer at baseline to collect additional information, including more detailed weight history. Of the 334,905 participants who completed the second questionnaire, we excluded participants whose baseline (n = 6,959) or second questionnaire (n = 3,424) were completed by proxy; those with self-reported prevalent cancer at baseline (n = 14,565) or any cancer diagnosis before the second questionnaire (n = 3,279); cancer only reported on death certificate (n = 2,451); and those with no information on weight at all ages (n = 607). Our final analytical cohort included 303,620 participants (176,789 men and 126,831 women). The study was approved by the Special Studies Institutional Review Board of the U.S. National Cancer Institute. All participants gave informed consent by virtue of completing and returning the questionnaire.
Exposure Assessment
The baseline questionnaire collected data on the participants’ current height and weight. The second questionnaire asked participants’ height at age 18 and weight at ages 18, 35, and 50. For individuals aged 50 at baseline, weights were collected for all four time points, but their weights at age 50 and at baseline had the same value. BMI at age 18 was derived using height and weight at age 18 while BMI at ages 35, 50, and at baseline were derived using weight at each respective age and height at baseline. BMI was categorized based on the current World Health Organization classifications (underweight, < 18.5 kg/m2; normal weight, 18.5 – < 25.0 kg/m2; overweight, 25 – < 30 kg/m2; obese class I, 30 – < 35 kg/m2; obese class II, 35 – < 40 kg/m2; obese class III, ≥ 40 kg/m2). Based on BMI at each age, we identified the time when BMI first exceeded 25 kg/m2 (never; by age 18; after 18 and by 35; after 35 and by 50; after 50 and by baseline).17 Furthermore, we calculated weight change between the four time points (age 18, 35, 50, and age at baseline) and categorized the amount of change as lost ≥ 2kg, lost < 2kg or gain < 5kg, gain 5 – < 15kg, or gain ≥ 15kg.18, 19
Identification of incident primary HCC and ICC
Diagnosis of HCC and ICC was ascertained through December 31, 2011, via linkage to state cancer registries of the eight baseline states plus three states where the study participants were most likely to relocate: Arizona, Nevada, and Texas. This approach has been estimated to have a sensitivity of 90% and a specificity of nearly 100%.20 HCC and ICC cases were identified using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) topography (C22) and morphology codes (8170–8175 for HCC; 8032, 8033, 8070, 8071, 8140, 8141, 8160, 8161, 8260, 8480, 8481, 8490, and 8560 for ICC).21
Statistical Analysis
Cox proportional hazards modeling was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) adjusted for confounders and covariates, using follow-up time as the underlying time-metric. In all analyses, person-time began on the date the second questionnaire was received and scanned, and ended on the date of first primary cancer diagnosis, death, end of follow-up, or the date the participant moved out of registry ascertainment area, whichever came first. This definition allowed us to censor participants with a first primary cancer diagnosis other than HCC or ICC, to ensure that we excluded secondary liver cancer as a result of metastasis from the primary site. The proportional hazards assumption was tested using likelihood ratio testing by comparing models with and without an interaction term between person-time and the main exposure variable. No violation was detected.
To identify distinct groups of participants with similarities in BMI trajectories, latent-class group-based trajectory models with maximum likelihood estimation were employed using a censored normal distribution,22 as implemented in SAS PROC TRAJ (SAS Institute, Cary, NC).23 As a basis for model selection, the number of groups and the order of the polynomial function were determined using the Bayesian information criterion (BIC).22 Models with 3–6 trajectories (requiring a minimum of 2% of participants per group) and linear, quadratic, or cubic order terms were considered.
All models were adjusted, a priori, for sex and age at study entry. Adjustment for physical activity, history of diabetes, alcohol drinking, smoking status, and consumption of red meat was also made because these variables changed the HR estimates by >10%. Weight change models additionally adjusted for the mean weight between the beginning and end of the period of change. This adjustment may produce more robust results than simply adjusting for the initial weight, because initial weight and weight change are not independent variables, thus may introduce bias.13 Other variables were examined but did not materially change the HR, included race, marital status, aspirin use, total energy intake, and coffee consumption. We tested for interaction of BMI with age at study entry (5-year age groups), sex, smoking (never, former, or current), alcohol consumption (quartiles), and history of diabetes (yes/no), using likelihood ratio tests.
Several sensitivity analyses were conducted, including 1) examining all primary liver cancer rather than HCC or ICC, as defined using topography (C22); 2) using age as the underlying time-metric; 3) constructing sex-specific BMI trajectories; and 4) excluding the first two years of follow-up to account for potential reverse causation (i.e., individuals can lose weight because of pre-clinical disease).
All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). All statistical tests were two sided, and p values < 0.05 were considered statistically significant.
RESULTS
The mean age of participants was 62.3 years at the administration of the baseline questionnaire (range 50.3–71.5 years). Fifty-eight percent of participants were men, and the great majority (>90%) were non-Hispanic white. Table 1 presents participants’ characteristics by baseline BMI. Compared to those with normal BMI, overweight or obese individuals were more likely to be male, have higher intakes of both total calories and red meat and have a history of diabetes. Conversely, they were less likely to have a college degree, be physically active, or be current smokers. Underweight individuals were more likely to be current smokers and less likely to be male. Individuals with higher BMI at baseline tended to have higher BMI at younger ages.
Table 1.
Participants’ characteristics according to body mass index (BMI) categories at baseline, the NIH-AARP study
| BMI at baseline (kg/m2) | ||||
|---|---|---|---|---|
|
| ||||
| < 18.5 (Underweight) | 18.5 – < 25 (Normal) | 25 – < 30 (Overweight) | ≥ 30 (Obese) | |
| N | 3,162 | 106,966 | 125,146 | 62,704 |
|
| ||||
| Age at baseline questionnaire, mean (SD) | 62.7 (5.4) | 62.4 (5.4) | 62.4 (5.3) | 61.7 (5.3) |
| Age at risk factor questionnaire, mean (SD) | 63.3 (5.4) | 63.0 (5.4) | 63.0 (5.3) | 62.2 (5.3) |
| Men, % | 38.1 | 49.2 | 68.1 | 56.2 |
| Race (non-Hispanic white), % | 91.9 | 93.4 | 92.7 | 90.9 |
| Alcohol intake, g/day, mean (SD) | 11.9 (31.6) | 12.7 (33.3) | 14.3 (37.4) | 11.4 (37.7) |
| Coffee intake, g/day, mean (SD) | 775.2 (691.4) | 747.7 (634.0) | 789.7 (631.6) | 747.5 (639.9) |
| Total red meat intake, g/day, mean (SD) | 54.2 (56.7) | 53.1 (55.9) | 68.3 (60.0) | 80.0 (73.1) |
| Total calories, kcal/day, mean (SD) | 1769.2 (863.6) | 1761.4 (851.7) | 1875.7 (889.4) | 1949.5 (1041.9) |
| College graduate, % | 43.8 | 45.1 | 41.4 | 33.9 |
| Took aspirin in the past 12 months, % | 65.8 | 71.1 | 74.5 | 70.8 |
| Currently smoking, % | 22.9 | 13.7 | 9.6 | 8.1 |
| Had a history of diabetes, % | 4.9 | 4.3 | 8.1 | 16.7 |
| Current moderate/vigorous physical activity > 7 hours/week, % | 27.2 | 28.6 | 23.6 | 15.1 |
| BMI at age 18, mean (SD) | 19.8 (2.7) | 20.3 (2.5) | 21.4 (2.9) | 23.0 (3.8) |
| BMI at age 35, mean (SD) | 18.9 (3.2) | 21.8 (2.5) | 24.0 (2.9) | 26.8 (4.4) |
| BMI at age 50, mean (SD) | 19.1 (3.7) | 22.5 (2.3) | 25.8 (2.6) | 30.7 (4.8) |
| BMI at baseline, mean (SD) | 16.9 (1.5) | 22.8 (1.5) | 27.2 (1.4) | 34.0 (4.0) |
Abbreviations: BMI, body mass index; SD, standard deviation.
During a mean follow-up of 11.9 (standard deviation 4.7) years, 372 HCC cases and 104 ICC cases were diagnosed. As shown in Table 2, being obese at age 18 was associated with higher risk of HCC (HR 1.86, 95% CI 1.06–3.27). Similarly, being obese at ages 35, 50 or at baseline were each associated with an approximately 2-fold elevated risk of HCC. A higher risk of ICC was associated with BMI at age 18 (HR 1.34, 95% CI 1.02–1.75, per 5 kg/m2), but not at ages 35, 50 or at baseline. In addition, we observed an increased risk of HCC and ICC among participants who were first overweight before age 18 or between ages 18 and 35, compared to those who were never overweight, but the associations attenuated for the participants who were first overweight after age 35 (Table 3).
Table 2.
Associations of body mass index (BMI) at various ages with incidence of HCC and ICC, the NIH-AARP study
| HCC incidence
|
ICC incidence
|
|||||
|---|---|---|---|---|---|---|
| Events, n | Person-years | Adjusted HR (95% CI)a | Events, n | Person-years | Adjusted HR (95% CI)a | |
| BMI, age 18, kg/m2 | ||||||
| < 18.5 | 53 | 499,041 | 1.09 (0.81, 1.47) | 14 | 499,041 | 1.03 (0.58, 1.83) |
| 18.5 – < 25 | 240 | 2,444,400 | Ref | 67 | 2,444,400 | Ref |
| 25 – < 30 | 38 | 302,849 | 0.99 (0.70, 1.39) | 15 | 302,849 | 1.74 (0.99, 3.07) |
| ≥ 30 | 13 | 52,560 | 1.86 (1.06, 3.27) | 3 | 52,560 | 2.03 (0.63, 6.50) |
| Per 5 kg/m2 increase | 1.10 (0.94, 1.29) | 1.34 (1.02, 1.75) | ||||
| BMI, age 35, kg/m2 | ||||||
| < 18.5 | 8 | 115,958 | 1.05 (0.52, 2.14) | 2 | 115,958 | 0.72 (0.18, 2.96) |
| 18.5 – < 25 | 167 | 2,287,351 | Ref | 56 | 2,287,351 | Ref |
| 25 – < 30 | 137 | 869,945 | 1.52 (1.21, 1.93) | 36 | 869,945 | 1.62 (1.04, 2.52) |
| ≥ 30 | 40 | 179,265 | 1.97 (1.37, 2.83) | 6 | 179,265 | 1.33 (0.56, 3.15) |
| Per 5 kg/m2 increase | 1.29 (1.14, 1.47) | 1.25 (0.97, 1.61) | ||||
| BMI, age 50, kg/m2 | ||||||
| < 18.5 | 4 | 69,402 | 1.02 (0.37, 2.76) | 3 | 69,402 | 1.66 (0.51, 5.35) |
| 18.5 – < 25 | 94 | 1,644,410 | Ref | 43 | 1,644,410 | Ref |
| 25 – < 30 | 181 | 1,329,569 | 1.70 (1.32, 2.20) | 39 | 1,329,569 | 1.03 (0.65, 1.61) |
| ≥ 30 | 77 | 433,640 | 2.06 (1.49, 2.84) | 15 | 433,640 | 1.23 (0.66, 2.31) |
| Per 5 kg/m2 increase | 1.35 (1.20, 1.51) | 1.15 (0.91, 1.45) | ||||
| BMI at baseline, kg/m2 | ||||||
| < 18.5 | 3 | 36,337 | 1.52 (0.48, 4.81) | 1 | 36,337 | 1.08 (0.15, 7.95) |
| 18.5 – < 25 | 74 | 1,285,644 | Ref | 32 | 1,285,644 | Ref |
| 25 – < 30 | 154 | 1,479,448 | 1.36 (1.02, 1.80) | 43 | 1,479,448 | 1.09 (0.68, 1.74) |
| ≥ 30 | 130 | 731,139 | 2.19 (1.62, 2.95) | 25 | 731,139 | 1.28 (0.74, 2.22) |
| Per 5 kg/m2 increase | 1.26 (1.13, 1.40) | 1.11 (0.90, 1.37) | ||||
Abbreviations: BMI, body mass index; CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma.
Adjusted for age at baseline, sex, physical activity, cigarette smoking, alcohol consumption, history of diabetes, and red meat consumption.
Table 3.
Associations of timing of being first overweight with incidence of HCC and ICC, the NIH-AARP study
| HCC incidence
|
ICC incidence
|
|||||
|---|---|---|---|---|---|---|
| Events, n | Person-years | Adjusted HR (95% CI)a | Events, n | Person-years | Adjusted HR (95% CI)a | |
| Time when body mass index first exceeded 25 kg/m2 | ||||||
| Never | 53 | 985,475 | Ref | 20 | 985,475 | Ref |
| By age 18 | 51 | 355,409 | 1.64 (1.11, 2.44) | 18 | 355,409 | 2.40 (1.24, 4.64) |
| By age 35 | 123 | 720,551 | 1.93 (1.38, 2.69) | 30 | 720,551 | 1.97 (1.09, 3.56) |
| By age 50 | 81 | 713,955 | 1.52 (1.07, 2.17) | 17 | 713,955 | 1.16 (0.60, 2.24) |
| By age at baseline | 27 | 438,726 | 0.93 (0.58, 1.48) | 13 | 438,726 | 1.38 (0.68, 2.78) |
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma.
Adjusted for age at baseline, sex, physical activity, cigarette smoking, alcohol consumption, history of diabetes, and red meat consumption.
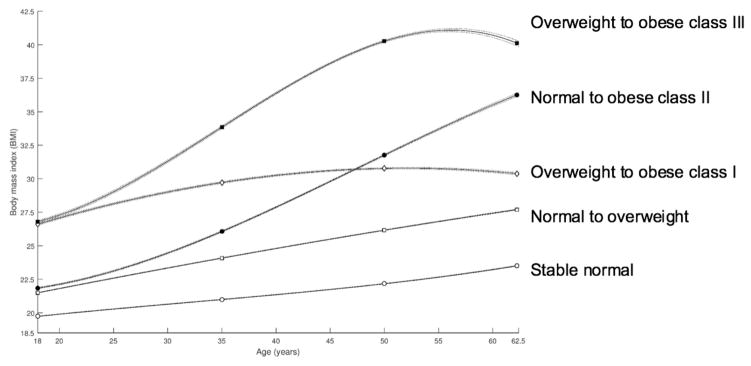
In trajectory analyses using cubic polynomial functions of age, five distinct trajectories of BMI during adulthood were identified (Figure 1). Thirty-seven percent of participants had a normal BMI throughout all ages studied, 46% had a normal BMI at age 18 and became overweight by baseline, 7% had an overweight BMI and became class I obese, 8% had a normal BMI at age 18 and became class II obese, while the remaining 2% had an overweight BMI and became class III obese (age-specific BMI for each trajectory is presented in Supplementary Table 1). Other combinations of BMI categories were not identified due to their low prevalence in the study population. As shown in Table 4, compared to the participants who had a normal BMI during the whole time period, HCC incidence was 28% higher for those with normal BMI and became overweight, and 71–84% higher for those whose trajectories resulted in any extent of obesity. The pattern for ICC was less clear, but there was a suggestion that the trajectories resulting in obese class II or III may be associated with over 50% higher ICC incidence.
Figure 1.
Trajectories of body mass index across adulthood among participants in the NIH-AARP study
Table 4.
Associations of body mass index (BMI) trajectories with incidence of HCC and ICC, the NIH-AARP study
| BMI trajectory | HCC incidence | ICC incidence | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Events, n | Person-years | Adjusted HR (95% CI)a | Events, n | Person-years | Adjusted HR (95% CI)a | |
| Stable normal | 85 | 1,341,465 | Ref | 33 | 1,341,465 | Ref |
| Normal to overweight | 190 | 1,694,816 | 1.28 (0.99, 1.67) | 51 | 1,694,816 | 1.14 (0.73, 1.79) |
| Normal to obese class II | 42 | 264,975 | 1.84 (1.25, 2.70) | 10 | 264,975 | 1.54 (0.74, 3.21) |
| Overweight to obese class I | 43 | 225,366 | 1.71 (1.17, 2.50) | 7 | 225,366 | 1.14 (0.49, 2.63) |
| Overweight to obese class III | 12 | 71,474 | 1.83 (0.98, 3.42) | 3 | 71,474 | 1.76 (0.52, 5.96) |
Abbreviations: BMI, body mass index; CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma.
Adjusted for age at baseline, sex, physical activity, cigarette smoking, alcohol consumption, history of diabetes, and red meat consumption.
Note: underweight, BMI < 18.5 kg/m2; normal weight, BMI 18.5 – < 25.0 kg/m2; overweight, BMI 25 – < 30 kg/m2; obese class I, BMI 30 – < 35 kg/m2; obese class II, BMI 35 – < 40 kg/m2; obese class III, BMI ≥ 40 kg/m2
In sensitivity analyses, the associations of BMI at each age and BMI trajectory with the risk of primary liver cancer were similar to what was observed with HCC in the main analysis (Supplementary Table 2). In addition, the evaluation of sex-specific BMI trajectories identified five trajectories in men (Supplementary Figure 1) and four trajectories in women (Supplementary Figure 2). Associations of sex-specific BMI trajectories with the incidence of HCC and ICC are shown in Supplementary Table 3. Analyses using age as the time scale or excluding the first 2 years of follow-up did not materially change the results. We did not observe effect modification by any factors on the association between BMI and the incidence of HCC or ICC (data not shown).
In a supplementary analysis of weight change, per 5 kg weight gain between ages 18–35 or 35–50 was associated with higher HCC incidence, and weight loss immediately before baseline was associated with higher HCC and ICC incidence (Supplementary Table 4).
DISCUSSION
The current study is the first prospective study on early adulthood adiposity in relation to both HCC and ICC, and the first to examine trajectories of adiposity change during adulthood on liver cancer risk. In this large prospective cohort, higher BMI at various ages across adulthood and BMI trajectories that resulted in obesity were associated with higher HCC incidence. The association between adiposity and ICC was less clear, although there was some indication that higher BMI at age 18 was associated with ICC development.
In age-specific obesity analysis, an approximate two-fold increased risk of HCC was observed across all ages studied. Previously, a case-control study (n = 622 cases and 660 controls) reported that obesity assessed at young adult ages was associated with two- to three-fold increased risk of HCC, similar to our estimates.11 Being overweight prior to study enrollment, especially at ages 35 or 50, was also associated with HCC in the current study. In addition, individuals who did not become overweight or obese until baseline (after age 50) had no elevated risk. Although our data did not allow us to determine the exact age at which adiposity starts to influence the risk of developing liver cancer, these findings collectively suggest that maintaining a healthy weight throughout the life course, especially in early adulthood, may be associated with lower risk of HCC.
Using a trajectory-based approach, five distinct groups of BMI evolution across adulthood were identified. The patterns closely resembled those previously observed in other U.S.-based studies.15, 18 Notably, individuals who were overweight in early adulthood and became class I obese had a ~70% higher risk of HCC, comparable to effect estimates for individuals who had a normal BMI and became Class II obese, suggesting that even if these individuals were only borderline obese at baseline (mean BMI 30.8 kg/m2), cumulative adiposity throughout adulthood may still impose a substantially increased risk of hepatocarcinogenesis.
In the current study, weight gain in early or mid-adulthood was associated with increased risk of HCC in each time period. Interestingly, participants with weight loss of over 2 kg between age 50 and baseline also had an elevated risk of HCC, which persisted after exclusion of the first 2 years of follow-up (data not shown). One likely explanation is that weight loss is a common symptom of cirrhosis 24 which may occur many years before HCC diagnosis. Thus, cirrhosis could potentially confound and attenuate the association between BMI and HCC incidence when adiposity is evaluated relatively close to the onset of HCC, whereas assessing weight in early adulthood before the onset of cirrhosis may provide a more robust estimate of this association.
Our study also examined the association between early obesity and ICC, which has not been previously reported. However, it has been suggested that HCC and ICC may share common risk factors, including obesity.25 Although the sample size was limited for our ICC analysis and most results did not reach statistical significance, there was a suggestion that elevated BMI at age 18 was associated with higher risk of ICC. Compared to results for HCC, ICC development seems to be influenced by adiposity at earlier stages of adulthood. Future studies with larger number of ICC cases are required to further elucidate this association.
Although not entirely understood, the mechanisms underlying the association between obesity and liver cancer, specifically HCC, are speculated to involve production of tumor-promoting cytokines and hepatic inflammation, altered gut microbial metabolites, as well as insulin resistance.26 Nonalcoholic fatty liver disease (NAFLD) is a common complication of obesity, including childhood and adolescence obesity.27, 28 The progression from NAFLD to steatohepatitis, fibrosis and cirrhosis may be involved in the obesity-induced hepatocarcinogenesis.29 As liver cancer takes several decades to develop, it is plausible that those with accumulation of excess liver fat at an earlier age are at increased risk of liver cancer. The mechanistic role of obesity in ICC is not well-studied, although it has been suggested that pro-inflammatory molecules, leptin, and adiponectin may be involved.30
There are several strengths of our study. It is the first to comprehensively evaluate trajectories of BMI during adulthood with the incidence of HCC and ICC in a large prospective cohort. We specifically examined the timing of being first overweight to evaluate if deviation from normal weight at an earlier age is more influential in liver cancer development. Collection of detailed covariate data enabled the assessment of potential confounders and important effect modifiers. This study also had several limitations. The study had no information on HBV or HCV infection status and the role of adiposity in hepatocarcinogenesis may differ between persons who are infected with HBV or HCV and persons who are not.31 In our analyses we adjusted for baseline smoking status, but we were unable to account for smoking across the life course due to the lack of this information. Exposure misclassification is possible, given that weight and height were self-reported, and information from ages 18, 35, and 50 was based on recall from earlier life periods. However, previous studies have shown that self-reported recalled past weight has a fairly high validity.32, 33 Furthermore, it has been reported that BMI distributions in the NIH-AARP cohort were similar to contemporaneous population data for the respective age group, suggesting that recall bias is not substantial.34 The NIH-AARP study mainly consisted of non-Hispanic white individuals, which limits the generalizability of our finding to other racial/ethnic groups, especially Hispanics and Blacks, among whom the prevalence of obesity35 and the liver cancer rates forecast for the next few decades36 are both high and prevention efforts are crucially needed.
In conclusion, higher BMI across adulthood and BMI trajectories that resulted in obesity may be associated with elevated HCC incidence. There is also a suggestion that adiposity may affect ICC development at an earlier age than HCC. Our results, if replicated in future studies, indicate that maintaining a healthy body weight throughout the life course may be important for the primary prevention of liver cancer. Given the rising incidence and mortality of primary liver cancer in many historically lower-risk areas 4 and the ongoing global epidemic of obesity,7 future studies (preferably studies with longitudinally collected height and weight information) are warranted to further elucidate the role of life-course adiposity in liver cancer development.
Supplementary Material
Novelty and Impact.
This is the first prospective study of early adulthood adiposity in relation to hepatocellular carcinoma and intrahepatic cholangiocarcinoma, and the first to examine trajectories of adiposity change during adulthood in relation to risk. Our findings suggest that maintaining a healthy body mass index throughout lifetime may be important for prevention of both outcomes.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under contract with the Florida Department of Health, Tallahassee, Florida. The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, The Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, Nevada.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis.
Abbreviations
- BIC
Bayesian information criterion
- BMI
body mass index
- CI
confidence interval
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- ICC
intrahepatic cholangiocarcinoma
- ICD-O-3
International Classification of Diseases for Oncology, 3rd edition
- NAFLD
nonalcoholic fatty liver disease
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; [accessed on 2015/07/23, 2013]. Available from: http://globocan.iarc.fr/ [Google Scholar]
- 2.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing Hepatocellular Carcinoma Incidence and Liver Cancer Mortality Rates in the United States. Am J Gastroenterol. 2014;109:542–53. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, Anderson RN, Ma J, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–37. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016;139:1534–45. doi: 10.1002/ijc.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223–38. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: An updated meta-analysis of prospective studies. Eur J Cancer. 2012;48:2137–45. doi: 10.1016/j.ejca.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization; World Health Organization, editor. Obesity: preventing and managing the global epidemic. 2000. [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;219:1–8. [PubMed] [Google Scholar]
- 9.Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF, McGlynn KA. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–65. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berentzen TL, Gamborg M, Holst C, Sørensen TIA, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol. 2014;60:325–30. doi: 10.1016/j.jhep.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, Hawk E, Morris J, Singh Raghav KP, Lee JS, Vauthey JN, Bortus G, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149:119–29. doi: 10.1053/j.gastro.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–88. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 13.Oldham PD. A note on the analysis of repeated measurements of the same subjects. J Chronic Dis. 1962;15:969–77. doi: 10.1016/0021-9681(62)90116-9. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, Boffetta P, Dahm CC, Overvad K, Tjonneland A, Halkjaer J, Fagherazzi G, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132:645–57. doi: 10.1002/ijc.27645. [DOI] [PubMed] [Google Scholar]
- 15.Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, Chan AT, Giovannucci EL. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138:2383–95. doi: 10.1002/ijc.29981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 17.Adams KF, Leitzmann MF, Ballard-Barbash R, Albanes D, Harris TB, Hollenbeck A, Kipnis V. Body mass and weight change in adults in relation to mortality risk. Am J Epidemiol. 2014;179:135–44. doi: 10.1093/aje/kwt254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly SP, Graubard BI, Andreotti G, Younes N, Cleary SD, Cook MB. Prediagnostic Body Mass Index Trajectories in Relation to Prostate Cancer Incidence and Mortality in the PLCO Cancer Screening Trial. J Natl Cancer Inst. 2017:109. doi: 10.1093/jnci/djw225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol. 2014;179:1353–65. doi: 10.1093/aje/kwu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaud D, Midthune D, Hermansen S, Leitzmann M, Harlan L, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Registry Manag. 2005;32:70–5. [Google Scholar]
- 21.Fritz AG, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin D. International Classification of Disease for Oncology: ICD-O. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 22.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods. 1999;4:139–57. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 23.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–71. [Google Scholar]
- 24.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagozian R, Derdak Z, Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63:607–17. doi: 10.1016/j.metabol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Barshop NJ, Francis CS, Schwimmer JB, Lavine JE. Nonalcoholic fatty liver disease as a comorbidity of childhood obesity. Ped Health. 2009;3:271–81. doi: 10.2217/phe.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond) 2009;33(Suppl 1):S60–5. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AMS. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Parsi MA. Obesity and cholangiocarcinoma. World J Gastroenterol. 2013;19:457–62. doi: 10.3748/wjg.v19.i4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–21. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 32.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6:61–6. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Klipstein-Grobusch K, Kroke A, Boeing H. Reproducibility of self-reported past body weight. Eur J Clin Nutr. 1998;52:525–8. doi: 10.1038/sj.ejcn.1600601. [DOI] [PubMed] [Google Scholar]
- 34.Renehan AG, Flood A, Adams KF, Olden M, Hollenbeck AR, Cross AJ, Leitzmann MF. Body mass index at different adult ages, weight change, and colorectal cancer risk in the National Institutes of Health-AARP Cohort. Am J Epidemiol. 2012;176:1130–40. doi: 10.1093/aje/kws192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.