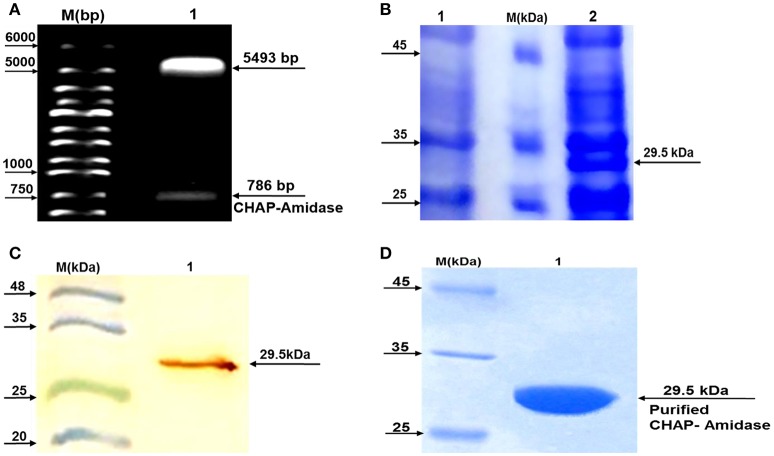
Figure 5.
Analysis of LysK-CHAP sequence by agarose gel electrophoresis and analysis of protein expression by SDS-PAGE and western blotting. (A) Lane 1: pET-22b/CHAP-amidase plasmid double digested by NcoI and HindIII restriction enzymes. Lane M: Molecular size marker (1 kb ladder). Arrows indicate the position of DNA fragments. The presence of a 786 bp fragment confirms the accuracy of the cloning of LysK-b/CHAP-amidase gene into pET-22 vector. (B) Expression analysis of CHAP-amidase derived from the soluble phase of E. coli cell lysate, by SDS-PAGE (10%). Lane 1: Bacterial lysate before induction by IPTG, M: Molecular weight markers (Fermentas Company), Lane 2: Bacterial lysate after induction by IPTG. (C) Western blot analysis of the expressed CHAP-amidase protein reacted with anti-His tag mAb. M: Pre-stained protein molecular weight markers, Lane 1: CHAP-amidase-expressing bacterial lysate. The arrow indicates the position of the overexpressed CHAP-amidase recombinant protein. (D) Purification of CHAP-amidase; M: Protein marker, 1: Purified protein.