Figure 3.
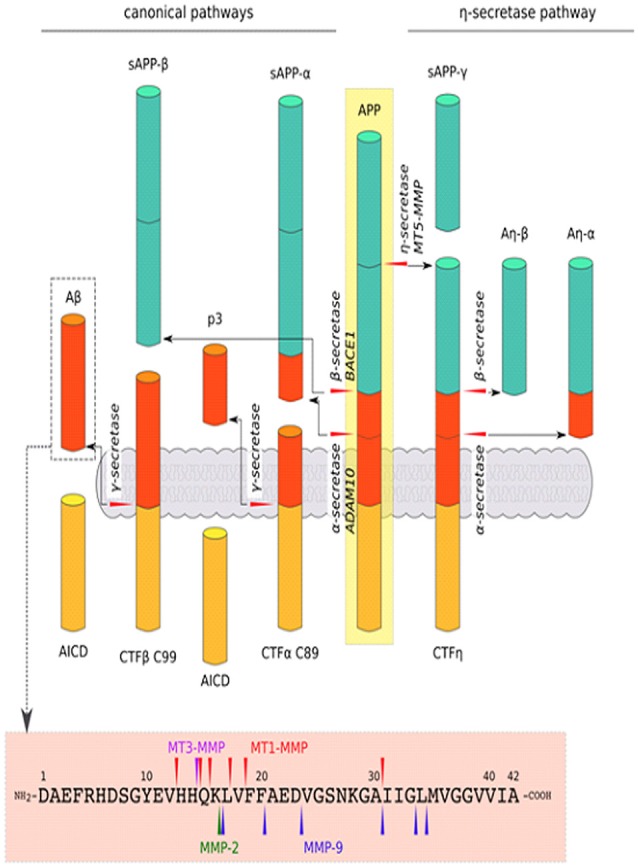
Schematic cleavage of APP in the canonical pathways by α-, β-secretase, a new cleavage pathway by η-secretase and Aβ1–42 degradation by MMPs: MMP-2, MMP-9, MT1-MMP and MT3-MMP. In the canonical pathway, APP is cleaved by β-secretase, generating the secreted ectodomain sAPP-β and membrane-associated C-terminal fragment (CTFβ) C99, which is then cleaved by γ-secretase to yield amyloid β (Aβ) peptide and the APP intracellular domain (AICD; i.e., amyloidogenic pathway). APP can also be cleaved by α-secretase in the canonical pathway. Cleavage by this mechanism results in sAPP-α and CTFα C89, which are further processed by γ-secretase to generate p3 and AICD (i.e., non-amyloidogenic pathway). Another mechanism, via η-secretase in the non-canonical pathway, yield sAPP-η and CTFη, which are further cleaved by α- and β-secretase to Aη-α and Aη-β fragments, respectively. Moreover, various proteinases, especially MMPs, that act beyond APP processing (described above) are involved in the direct degradation of Aβ peptide (O’Brien and Wong, 2011; Dawkins and Small, 2014; Zhang et al., 2015; Andrew et al., 2016; Wang Y. Q. et al., 2016).
