Figure 1.
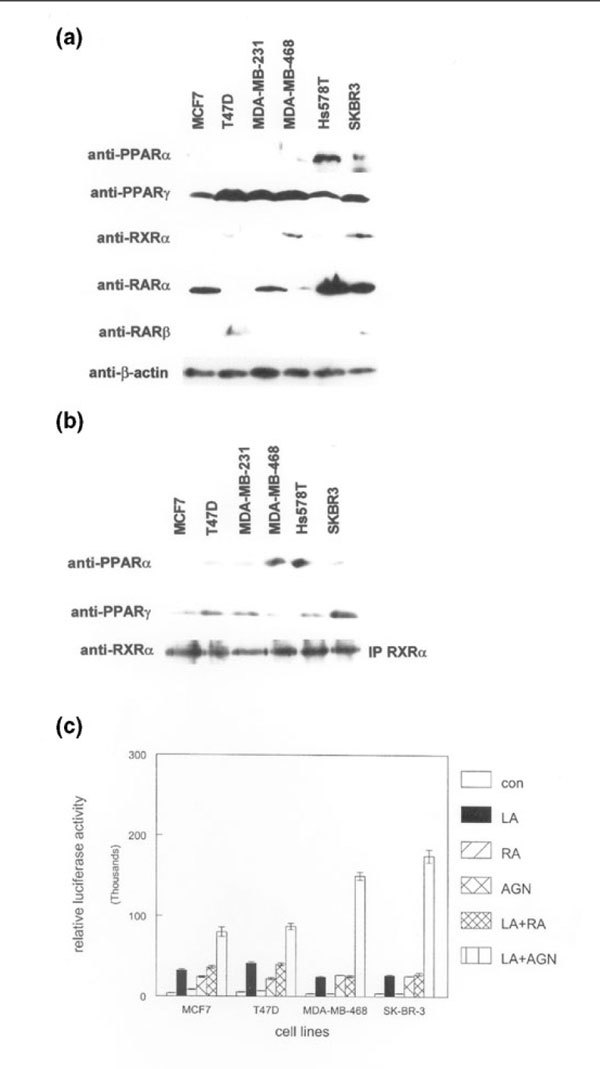
Expression of functionally interacting peroxisome proliferator-activated receptors (PPARs), retinoic acid receptors (RARs), and retinoid X receptors (RXRs) in human breast cancer cell lines. (a) Whole cell lysates from the indicated breast cancer cell lines were subjected to western blot analysis using the anti-PPAR, anti-RAR, anti-RXR, and anti-β-actin antibodies shown on the left. Relative expression of each protein was determined using the same membrane. (b) Human RXR-α protein was immunoprecipitated (IP RXRα) from the indicated breast cancer cell lines. Immunoprecipitated complexes were subjected to western blotting using anti-PPAR-α and anti-PPAR-γ antibodies. Blots were stripped and incubated with anti-RXR-α antibody to determine the relative amounts of immunoprecipitated protein in each lane. These experiments were performed three times, with similar results. Representative blots are shown. (c) An RXR-selective compound potentiates transcriptional activation by a PPAR ligand. The indicated human breast cancer cell lines were transiently transfected with a heterologous PPAR-responsive promoter/reporter construct and treated with 100 μmol/l of the PPAR ligand γ-linolenic acid (LA), 100 nmol/l of the pan RAR agonist all-trans retinoic acid (RA), 100 nmol/l of the RXR-selective ligand AGN194204 (AGN), γ-linolenic acid and retinoic acid (LA+R), or γ-linolenic acid plus AGN194204 (LA+A) for 24 hours before determination of promoter activity. Promoter activity was represented as relative light units from the luciferase reporter. These experiments were performed three times with similar results. Error bars indicate the standard error of the mean.
