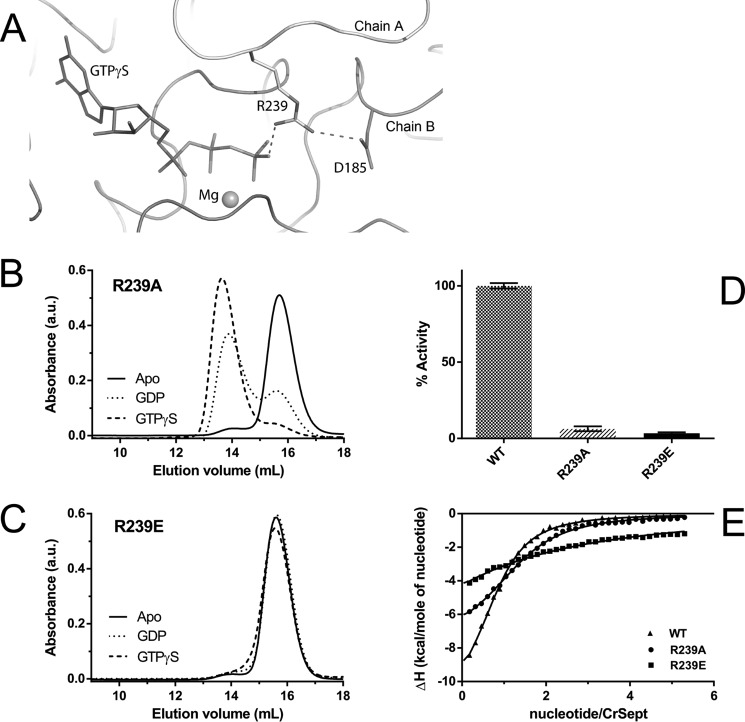
Figure 4.
GTPase Activity of CrSEPT employs a catalytic arginine finger. A, hydrogen-bond interaction between Arg-239 (chain B) and the γ-phosphate of GTPγS within the CrSEPT nucleotide-binding pocket of chain A. The Arg-239 also interacts via a hydrogen bond with Asp-185 (from the switch II region). The magnesium is shown as a sphere. B and C, influence of the nucleotide on the oligomeric state of the R239A and R239E mutants. The mutation to Ala (R239A) did not influence GTP-dependent dimerization, whereas substitution by Glu (R239E) completely prevented dimerization. D, both mutants were unable to hydrolyze GTP. E, GTPγS binding affinities for CrSEPT mutants were determined using ITC, as in Fig. 2A. The following Kd values were obtained from the fits: R239A, 7.6 ± 0.4 μm; R239E, 103 ± 4 μm; WT, 5.4 ± 0.3 μm. Experiments were performed in triplicate, and the mean and S. D. values are reported.