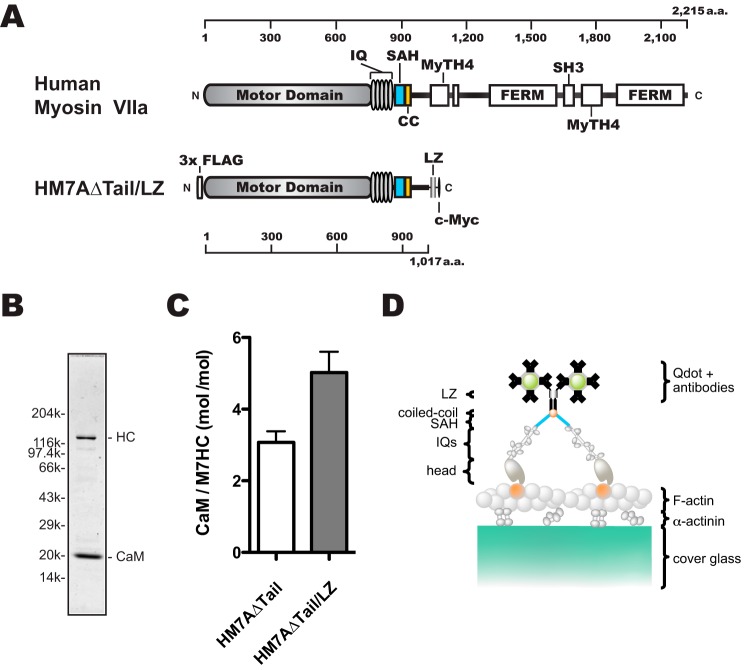
Figure 1.
Schematic diagrams of human myosin VIIa dimer construct (HM7AΔTail/LZ) used in this study. A, schematic diagram of human myosin VIIa construct. Upper, human myosin VIIa (1–2215 amino acids). Lower, forced dimer of human myosin VIIa (without tail domain) construct. The construct consisted of 3× FLAG, motor domain, 5× IQ motifs, an SAH domain, and LZ and c-Myc domains. The amino acid (a.a.) numbers of human myosin VIIa are shown at the top and bottom in A. B, SDS-PAGE of the purified HM7AΔTail/LZ. The myosin VIIa heavy chain was co-expressed with calmodulin and purified with anti-FLAG antibody agarose. Note that purified HM7AΔTail/LZ contains exogenous calmodulin that is added through all of the purification steps. Right: HC, HM7AΔTail/LZ heavy chain. Left, molecular mass markers described under “Experimental Procedures.” C, stoichiometry of CaM bound to HM7AΔTail and HM7AΔTail/LZ. Molar ratios of calmodulin: HM7AΔTail and HM7ADTail/LZ were estimated as described under “Experimental Procedures.” The means ± S.E. were 3.1 ± 0.3 (n = 4) and 5.0 ± 0.6 (n = 6), respectively. The p value of unpaired t test was 0.03 between HM7AΔTail and HM7AΔTail/LZ. D, schematic diagram of TIRF motility assay. HM7AΔTail/LZ labeled with Qdot525 is shown on an actin filament attached to α-actinin-coated cover glass. It is anticipated that HM7AΔTail/LZ dimerizes at the LZ motif with two Qdots at the maximum.