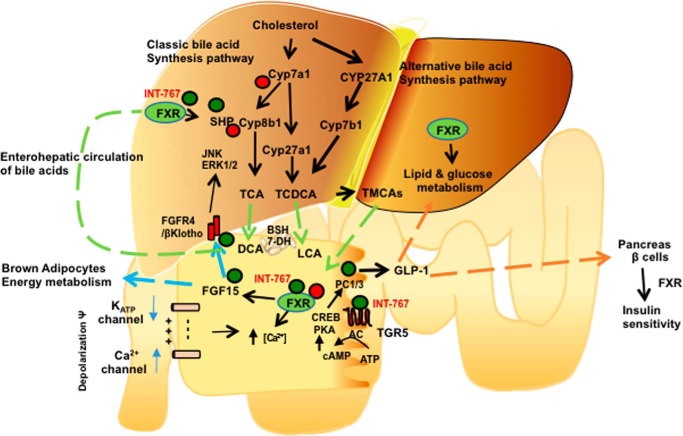
Figure 9.
Mechanisms of intestinal FXR and TGR5 cross-talk in the regulation of GLP-1 secretion, hepatic bile aid synthesis, glucose and lipid metabolism, and insulin and glucose sensitivity. In the liver, the classic bile acid synthesis is initiated by cholesterol 7α-hydroxylase (Cyp7a1 and Cyp8b1 catalyze cholic acid synthesis, and sterol 27-hydroxylase catalyzes steroid side-chain oxidation to synthesize CA and CDCA, which are conjugated to taurine (T)). The alternative pathway is initiated by Cyp27a1 and oxysterol 7α-hydroxylase (Cyp7b1) to synthesize mainly CDCA. INT-767 activates FXR/SHP in hepatocytes to repress Cyp7a1 and Cyp8b1 and stimulate Cyp7b1 and Cyp27a1 expression to improve glucose and lipid metabolism. In intestinal L cells, INT-767 activates FXR, which stimulates TGR5 and prohormone convertase 1/3 (PC1/3) expression. FXR also induces FGF15, which stimulates energy metabolism in brown adipocytes. FGF15 activates hepatic FGFR4/βKlotho signaling to inhibit Cyp7a1 via JNK/ERK1/2 pathway. TGR5 activation stimulates adenylyl cyclase (AC) to increase cAMP, which activates protein kinase A (PKA) and phosphorylates and activates CREB to increase PC1/3 transcription. PC1/3 splices pre-proglucagon to GLP-1. INT-767 increases intracellular [Ca2+] and cAMP activity. INT-767 activation of FXR may depolarize membrane potential by inhibiting KATP channel to stimulate Ca2+ uptake through Ca2+ channels. Ca2+ stimulates GLP-1 secretion from L cells. GLP-1 and FXR stimulate insulin synthesis and secretion from pancreatic β cells.