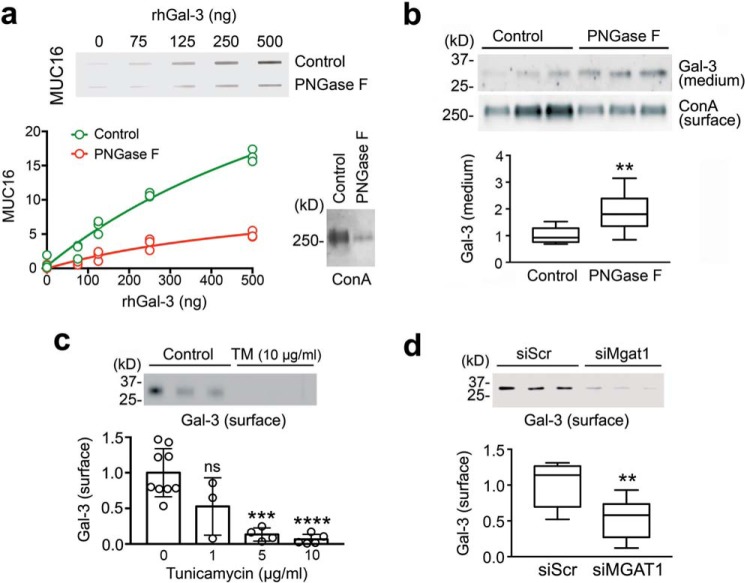
Figure 6.
Galectin-3 interaction with MUC16 and its retention on the cell surface were N-glycan-dependent. a, 2-fold serial dilutions of rhGal-3 were applied individually to a nitrocellulose membrane in a slot-blot apparatus. Membranes were subsequently incubated with denatured cell lysates treated with or without PNGase F for 3 h at 37 °C. The enzymatic release of N-glycans significantly reduced the binding activity of MUC16 toward immobilized rhGal-3. Removal of N-glycans after PNGase F treatment was evaluated using ConA. b, treatment of stratified human corneal epithelial cells with PNGase F for 24 h significantly increased the amount of galectin-3 present in the cell culture medium. Relative amounts of N-glycans in biotinylated cell-surface proteins were evaluated using ConA. c, stratified human corneal epithelial cells were cultured in medium containing 1–10 μg/ml tunicamycin (TM) for the last 3 days of culture. Cells were then surface-labeled with biotin at 4 °C and pulled down using NeutrAvidin™. Galectin-3 was detected by Western blotting. d, cultures of human corneal epithelial cells were transfected with either non-targeting scramble control (siScr) or MGAT1-targeting siRNA (siMGAT1). As observed by cell-surface biotinylation and Western blotting, the knockdown of MGAT1 decreased the abundance of cell-surface galectin-3. Results in a and c represent at least three independent experiments. Results in b and d represent three independent experiments performed in triplicate. Data in c are represented as the mean ± S.D. The box and whisker plot show the 25 and 75 percentiles (box) and the median and the minimum and maximum data values (whiskers). Significance was determined using Student's t test (b and d) and one-way analysis of variance with Tukey's post hoc test (c). **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, nonsignificant.