Abstract
Key points
Large conductance, Ca2+‐activated K+ (BKCa) channels play important roles in mammalian retinal neurons, including photoreceptors, bipolar cells, amacrine cells and ganglion cells, but they have not been identified in horizontal cells.
BKCa channel blockers paxilline and iberiotoxin, as well as Ca2+ free solutions and divalent cation Cav channel blockers, eliminate the outwardly rectifying current, while NS1619 enhances it. In symmetrical 150 mm K+, single channels had a conductance close to 250 pS, within the range of all known BKCa channels.
In current clamped horizontal cells, BKCa channels subdue depolarizing membrane potential excursions, reduce the average resting potential and decrease oscillations. The results show that BKCa channel activation puts a ceiling on horizontal cell depolarization and regulates the temporal responsivity of the cells.
Abstract
Large conductance, calcium‐activated potassium (BKCa) channels have numerous roles in neurons including the regulation of membrane excitability, intracellular [Ca2+] regulation, and neurotransmitter release. In the retina, they have been identified in photoreceptors, bipolar cells, amacrine cells and ganglion cells, but have not been conclusively identified in mammalian horizontal cells. We found that outward current recorded between −30 and +60 mV is carried primarily in BKCa channels in isolated horizontal cells of rats and mice. Whole‐cell outward currents were maximal at +50 mV and declined at membrane potentials positive to this value. This current was eliminated by the selective BKCa channel blocker paxilline (100 nm), iberiotoxin (10 μm), Ca2+ free solutions and divalent cation Cav channel blockers. It was activated by the BKCa channel activator NS1619 (30 μm). Single channel recordings revealed the conductance of the channels to be 244 ± 11 pS (n = 17; symmetrical 150 mm K+) with open probability being both voltage‐ and Ca2+‐dependent. The channels showed fast activation kinetics in response to Ca2+ influx and inactivation gating that could be modified by intracellular protease treatment, which suggests β subunit involvement. Under current clamp, block of BKCa current increased depolarizing membrane potential excursions, raising the average resting potential and producing oscillations. BKCa current activation with NS1619 inhibited oscillations and hyperpolarized the resting potential. These effects underscore the functional role of BKCa current in limiting depolarization of the horizontal cell membrane potential and suggest actions of these channels in regulating the temporal responsivity of the cells.
Keywords: K channel, mammalian retina, outward rectifier
Key points
Large conductance, Ca2+‐activated K+ (BKCa) channels play important roles in mammalian retinal neurons, including photoreceptors, bipolar cells, amacrine cells and ganglion cells, but they have not been identified in horizontal cells.
BKCa channel blockers paxilline and iberiotoxin, as well as Ca2+ free solutions and divalent cation Cav channel blockers, eliminate the outwardly rectifying current, while NS1619 enhances it. In symmetrical 150 mm K+, single channels had a conductance close to 250 pS, within the range of all known BKCa channels.
In current clamped horizontal cells, BKCa channels subdue depolarizing membrane potential excursions, reduce the average resting potential and decrease oscillations. The results show that BKCa channel activation puts a ceiling on horizontal cell depolarization and regulates the temporal responsivity of the cells.
Abbreviations
- BAPTA
1,2‐bis(o‐aminophenoxy)ethane‐N,N,N′,N′‐tetraacetic acid
- BKCa
big calcium‐activated potassium channel
- Cav channel
voltage‐gated calcium channel
- DMSO
dimethyl sulfoxide
- EGTA
ethylene glycol‐bis(β‐aminoethyl ether)‐N,N,N′,N′‐tetraacetic acid
- erg
ether‐à‐go‐go‐related gene
Introduction
Horizontal cells are non‐spiking inhibitory interneurons in the vertebrate retina that receive direct excitatory glutamatergic input from photoreceptors and make inhibitory feedback output onto photoreceptors and feed forward inhibition onto bipolar cells (Thoreson & Mangel, 2012; Liu et al. 2013; Hirano et al. 2016). Input from many photoreceptors is collected by the electrically coupled network of horizontal cells, each of which has a laterally broad dendritic field. The breadth of the horizontal cell receptive field results in their contribution of an antagonistic surround to the receptive field structures common to retinal neurons. Since they receive excitatory glutamatergic input from numerous photoreceptors, horizontal cells hyperpolarize in response to light in a manner that is graded with the intensity and spatial extent of the light stimulus. This means that in darkness, especially immediately after cessation of bright light, when photoreceptors are depolarized and release glutamate maximally, horizontal cells are also maximally depolarized, posing possible challenges to cellular homeostasis.
We sought to define the properties of a newly defined conductance in these cells, which we show is produced by large conductance, calcium‐activated potassium (BKCa) channels that act to oppose horizontal cell depolarization. Such an action not only keeps the horizontal cell membrane potential in the physiological operating range, limiting Ca2+ overloads, but also contributes to the frequency tuning of horizontal cells by regulating membrane potential oscillatory states. Currents with similar properties have been reported in mouse horizontal cells but the underlying channels were not identified as the BKCa type (Feigenspan et al. 2009). Previously, voltage‐ and calcium‐dependent single channels with a conductance of 138 pS were observed in inside‐out patches from cultured rabbit horizontal cells recorded under asymmetrical, physiological conditions, but a macroscopic current with the properties of BKCa channels was not detected in whole‐cell recordings in that report (Löhrke & Hofmann, 1994). In whole‐cell recordings from isolated cat horizontal cells, outward currents attributable to BKCa channels were also not detected over the limited voltage range tested and frequent superfusion of Co2+ (Ueda et al. 1992). These reports do not rule out the presence of BKCa channels, in general, in mammalian horizontal cells.
Outwardly rectifying BKCa channels are found in many neurons where they serve a diverse set of functions including action potential repolarization, membrane potential oscillatory frequency modulation and neurotransmitter release regulation (Lee & Cui, 2010). In the cochlear hair cells of many vertebrates, intrinsic electrical tuning to a narrow band of stimulus frequencies arises in part from the interaction between BKCa channels and voltage‐gated Cav channels (Pyott & Duncan, 2016).
We investigated the nature of the channels underlying outward rectification in horizontal cells from rat and mouse retina. We found that the majority of outward current between −30 and +80 mV is carried in BKCa channels. BKCa channels were defined by their large single channel conductance, their pharmacological sensitivity, which includes block by several highly specific BKCa channel blockers (iberiotoxin, paxilline) (Candia et al. 1992; Sanchez & McManus, 1996) as well as activation by the specific BKCa channel activator NS1619 (Olesen et al. 1994; Wrzosek, 2014), and their Ca2+ dependence, which endows the whole‐cell conductance with a bell‐shaped current–voltage (I–V) relation. We show that the BKCa channels have fast activation kinetics that coincide with the dynamics of Ca2+ influx and that their differential responses to [Ca2+]i buffering by ethylene glycol‐bis(β‐aminoethyl ether)‐N,N,N′,N′‐tetraacetic acid (EGTA) and 1,2‐bis(o‐aminophenoxy)ethane‐N,N,N′,N′‐tetraacetic acid (BAPTA) suggest that the channels are closely coupled to Ca2+ channels. Their rapid inactivation kinetics, which are sensitive to intracellular protease action, imply that the composition of these BKCa channels includes inactivation‐promoting β subunits (Hicks & Marrion, 1998), which may support frequency tuning properties of horizontal cells.
Methods
Ethical approval
Electrophysiological, imaging and immunohistochemical experiments were performed at UCLA in accordance with the guidelines for the welfare of experimental animals issued by the US Public Health Service Policy on Human Care and Use of Laboratory Animals and the University of California, Los Angeles Office of Animal Research Oversight.
Cell dissociation
Sprague–Dawley rats or Cx57‐tdTomato C57BL/6 mice (Hirano et al. 2016) of 3–5 weeks’ age were deeply anesthetized with isoflurane and killed by decapitation. The eyes were removed and placed in ice‐cold Hanks’ balanced salt solution (HBSS) and retinas were dissociated in Ca2+‐ and Mg2+‐free HBSS. Retinas were transferred into 10 ml Hibernate A medium containing 126 u ml−1 deoxyribonuclease and 1.5 mg ml−1 bovine serum albumin (BSA) following incubation in 13 u ml−1 papain (Worthington, Lakewood, NJ, USA) dissolved in Ca2+‐ and Mg2+‐free HBSS at 37°C for 30 min. The enzymatically treated retinas were gently triturated with a fire‐polished Pasture pipette and then plated on glass coverslips, which were pre‐coated with 0.1 mg ml−1 Con A. Cells adhered to the coverslips and were ready for patch clamping about 1 h after plating.
Electrophysiology
Ionic currents were studied using whole‐cell and single channel voltage‐clamp techniques. Patch electrodes were fabricated from borosilicate glass (0.86/1.5 mm in inner/outer diameter) with a Flaming–Brown horizontal puller (P‐87, Sutter Instruments, Novato, CA, USA). Electrodes were fire‐polished when used in single channel recording. After filling, electrodes had a final resistance of 5–7 MΩ for whole‐cell and 8–12 MΩ for single channel recording.
Whole‐cell recording
Whole‐cell recording was done as previously described (Sun et al. 1999). Patch electrodes were filled with internal solution containing (mm): 130 potassium gluconate, 20 KCl, 2 MgATP, 0.3 NaGTP, 5 sodium phosphocreatine, 1 EGTA and 10 Hepes, pH 7.2. In some experiments, potassium gluconate was replaced with equimolar KCl. For calcium current recordings, the internal solution contained (mm): 80 caesium methanesulfonate, 40 CsCl, 20 TEACl, 0.5 CaCl2, 1 MgCl2, 5 EGTA, 2 Na2ATP, 0.5 NaGTP and 10 Hepes, pH 7.2. In some experiments, caesium methanesulfonate was replaced with equimolar CsCl. After the formation of a gigaohm seal (seal resistance, > 4 GΩ), cells with access resistance of < 25 MΩ were used for study. Capacitance and series resistance were optimized and compensated by ∼60%. No leak subtraction was used. Typical cell membrane capacitances (C m) were 6–12 pF. The bath solution for whole‐cell or outside‐out patch recordings contained (in mm): 138 NaCl, 5 KCl, 1 MgCl2, 2.4 CaCl2, 1.25 NaH2PO4, 10 glucose and 10 Hepes, pH 7.3. In Ca2+‐deficient solution, equimolar MgCl2 was used to substitute for the reduced Ca2+. To record Ca2+ currents, 0.5–1 μm TTX was usually added to the bath solution. All bath solutions were bubbled with 95% O2–5% CO2 carbogen and superfused during the experiments. Voltage and current experiments were performed with an Axopatch Multiclamp amplifier (Molecular Devices, Sunnyvale, CA, USA) at room temperature. Pulse generation, data acquisition and analysis were done with a PC equipped with a Digidata 1322A interface in conjunction with pClamp 9.0 (Molecular Devices). Currents were filtered with a 4‐pole Bessel filter at 5 kHz. Unless otherwise specified, the holding potentials were −70 mV.
Single channel recording
Single‐channel currents were recorded in outside‐out and inside‐out patch configurations. Inside‐out patches were obtained by withdrawing patch pipettes from horizontal cells after formation of a gigaohm seal. To prevent the formation of a vesicle, patches were usually exposed to air briefly. The bath solution contained (in mm): 150 KCl, 10 Hepes, variable amounts of CaCl2 (see figure legends) and MgCl2 and 5 mm Ca2+ buffers to give different free [Ca2+] and 1 MgCl2. The amount of CaCl2 added was estimated by the program Slider 1.0 (created by C. Patton, Stanford University, CA, USA). In outside‐out patches, single channel recordings were made with bath solution after excision from whole‐cell patches. Single‐channel currents were filtered at 2–5 kHz with an Axopatch Multiclamp amplifier and sampled at 50 kHz using pCLAMP 9.0 software.
Single channel data analysis
The number of single channels in each patch was determined by fitting the channel current histogram to a Gaussian distribution (pClamp 10). Open probability (P o) was calculated using Clampfit 9.0 and expressed as:
where N is the number of channels in the patch and Pi the probability that i channels open simultaneously. The activation curves of single channel data are fit to a Boltzmann equation:
where V 0.5 is the voltage of half‐maximal activation of conductance, and k is the slope factor for the voltage dependence of the activation process.
Data analysis and statistics
Data are expressed as means ± standard error of the mean (SEM) with n indicating the number of recordings. Quantitative comparison between treatments was judged to be significant if P < 0.05 after analysis using two‐way ANOVA with the Bonferroni test or Student's t test.
Chemicals
BAPTA‐AM, EGTA‐AM and paxilline were obtained from Sigma‐Aldrich (St Louis, MO, USA). Iberiotoxin and tetrodotoxin were obtained from CalBiochem (San Diego, CA, USA). NS1619 was from Bio‐Techne (Minneapolis, MN, USA). All drugs were dissolved in bath solution and applied to the cells by superfusion.
Results
Horizontal cells were isolated from rat and mouse retina, and whole‐cell and single channel patch‐clamp recordings were made to investigate the gating and pharmacological properties of outward currents carried in BKCa channels. Horizontal cells from rat were identified on the basis of their characteristic morphology, which included a cell body of 11–15 μm diameter with a tuft of processes (Fig. 1 Aa). For this work, we identified rat horizontal cells on the basis of the size of the cell body and the presence of their characteristic and numerous small multipolar processes. The long axon and its terminal were invariably lost during isolation (Peichl & Gonzalez‐Soriano, 1994). Horizontal cells from mice were positively identified using fluorescence microscopy since the cells were isolated from a Cx57‐tdTomato transgenic mouse line in which horizontal cells strongly express the tdTomato fluorescent marker. An example of one of these fluorescing isolated cells is shown in Fig. 1 Ab–d).
Figure 1. Whole‐cell currents recorded from isolated horizontal cells of rat and mouse retina.
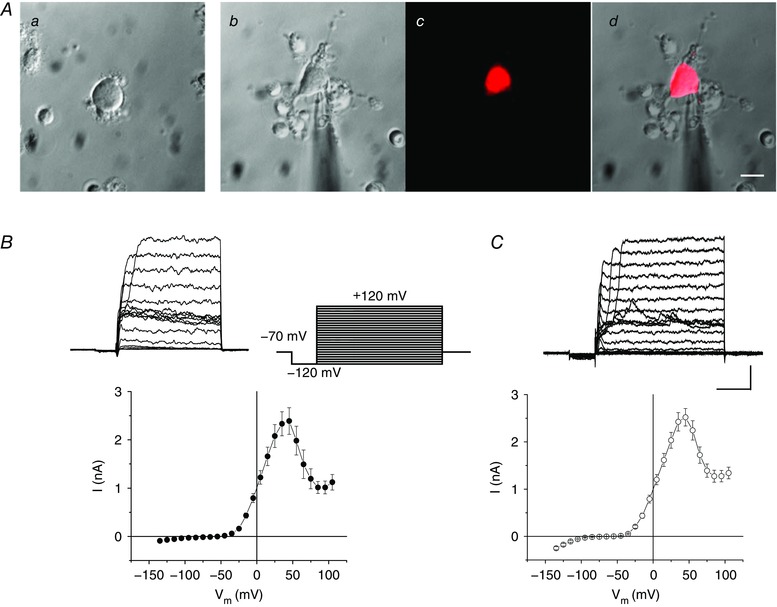
A, micrographs showing isolated horizontal cells from rat (a) and the Cx57‐tdTomato transgenic mouse obtained in differential interference contrast (b), fluorescence (c) and in a merged image (d). Scale bar: 10 μm. B, whole‐cell current elicited by a set of depolarizing voltage steps (inset to right) and I–V relationship (lower) in rats; n = 22 cells. C, similar current and I–V curve recorded in Cx57‐tdTomato mice; n = 24 cells. Scale bar: 1 nA, 20 ms. In Figs 2, 3, 4 and 7 following below, the voltage‐clamp protocol for whole‐cell recording is the same as shown here. [Color figure can be viewed at wileyonlinelibrary.com]
Voltage‐dependent activation of outward current
Whole‐cell currents were recorded from enzymatically isolated horizontal cells of rat (Fig. 1 B) and Cx57‐tdTomato transgenic mouse retina (Fig. 1 C). In both species, voltage steps elicited large outward currents positive to −30 mV and showed similar kinetics. For rat and mouse, the current–voltage (I–V) relations were nearly identical, each with outward current activating positive to −30 mV, a peak in this current near +50 mV, and then a decline in outward current positive to this value, reaching a nearly constant value at +70 to +80 mV.
Whole‐cell voltage‐clamp recordings showed strongly rectifying outward currents with a bell‐shaped I–V relationship in 53 and 98 horizontal cells dissociated from 16 rats and 27 mice, respectively. We also recorded single channel currents that had gating properties that showed closely related dependence. For comparison, Fig. 2 A shows a family of currents elicited during depolarizing steps and the resulting typical bell‐shaped whole‐cell I–V relation recorded from a Cx57 mouse horizontal cell. When a membrane patch was excised from this cell, forming an outside‐out patch (Fig. 2 B), single channel currents of very large conductance (∼115 pS; Fig. 2 C; asymmetrical K+ concentrations) were observed with high open probability between −25 mV and +55 mV (Fig. 2 D), approximately the same voltage range as seen for the bell‐shaped whole‐cell outward current recording. These observations suggest the likelihood that the whole‐cell outward current resulted from activation of BKCa channels in response to Ca2+ influx through voltage‐gated Cav channels over the same voltage range.
Figure 2. A large conductance single channel has voltage‐dependent characteristics matching the whole‐cell outward current in Cx57‐tdTomato mouse horizontal cells.
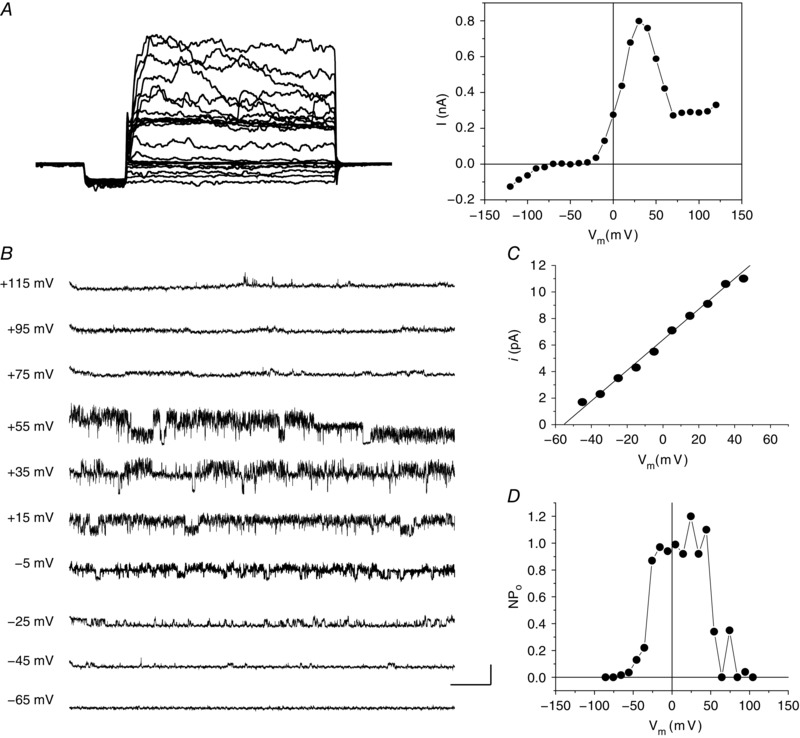
A, whole‐cell currents (left) elicited by depolarizing voltage steps (stimulus protocol as per Fig. 1) and the I–V relationship obtained from these steps (right). B, representative single channel current traces at different voltages from an outside‐out patch after excision from the same cell; 5 mm Ca2+ in bath solution. C, single channel I–V relationship with slope conductance of 115.6 pS calculated from a linear fit. D, open probability (NP o) was plotted against holding potential. Note that the bell‐shaped single channel NP o curve matches the whole‐cell current–voltage relation. Scale bar: 10 pA, 100 ms.
Outward current is sensitive to BKCa channel modulating agents
To test the hypothesis that the outward current was due to the activation of BKCa channels, we examined the effect of superfusing a Ca2+‐free solution on the current (Fig. 3 A). Ca2+‐free solution selectively reduced outward current between −30 and +80 mV. Figure 3 B shows that at its peak (∼+50 mV), normalized outward current was reduced from 78 ± 8% in control to 37 ± 13% in Ca2+‐free solution, without significantly affecting the current positive to +80 mV (47 ± 6% in control and 42 ± 4% in Ca2+‐free at +100 mV; P < 0.001, n = 6). Iberiotoxin (100 nm), a selective BKCa channel blocking toxin (Candia et al. 1992), also suppressed the current in a manner similar to that seen in Ca2+‐free solution (Fig. 3 C and D). The BKCa channel agonist NS1619 (30 μm) (Olesen et al. 1994; Wrzosek, 2014) increased normalized outward currents by 62 ± 15% at 0 mV and 40 ± 16% at +50 mV; Fig. 3 E and F). NS1619 also eliminated the bell‐shaped curve, preventing the currents from decaying positive to +50 mV, such that normalized currents at +100 mV were increased to 149 ± 39% of control peak values (P < 0.0001, n = 14).
Figure 3. Pharmacology of outward currents in Cx57‐tdTomato mouse horizontal cells reflects properties of a large conductance Ca2+‐activated K+ current.
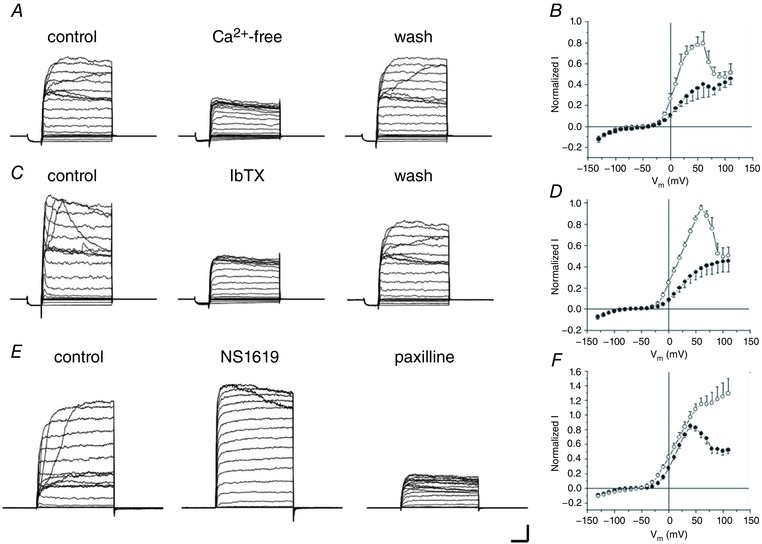
A, sample currents recorded before (control), during surperfusion of Ca2+‐free bath solution (Ca2+‐free) and after washing (wash) with normal bath solution. B, summarized results of I–V relationship of control (open circles) and Ca2+‐free (filled circles; n = 6). Currents were normalized to the peak control current for each neuron. C, current recordings before (control) and during 100 nm iberiotoxin (IbTX) superfusion and after washing (wash). D, summarized normalized I–V relation curves of control (open circles) and IbTX (filled circles; n = 7). E, example currents before (control), during 30 μm NS1619 superfusion, and following addition of 2.5 μm paxilline. F, summarized I–V curves of control (filled circles) and NS1619 (open circles; n = 14). Scale bar: 1 nA, 20 ms. Stimulus protocols as per Fig. 1.
To gain pharmacological evidence that these currents were carried in BKCa channels, as well as to estimate the contribution of this current to the total outward current in horizontal cells, we applied the BKCa channel blocker paxilline (Sanchez & McManus, 1996) to cells isolated from rats and mice. Paxilline (2.5 μm) blocked a large portion of outward current in both rats and mice, suggesting that about 60% and 80% of total outward current in these species, respectively, was BKCa current (Fig. 4 A–C). At the single channel level, paxilline also blocked currents in an outside‐out patch from a mouse horizontal cell (Fig. 4 D). Following activation of whole‐cell BKCa currents with the specific agonist NS1619, paxilline abolished all of the increased outward current as well as the control outward current, suggesting there were no other currents involved in effects of NS1619 (Fig. 3 E).
Figure 4. Effects of BKCa channel blocker paxilline on whole‐cell outward currents and single channels.
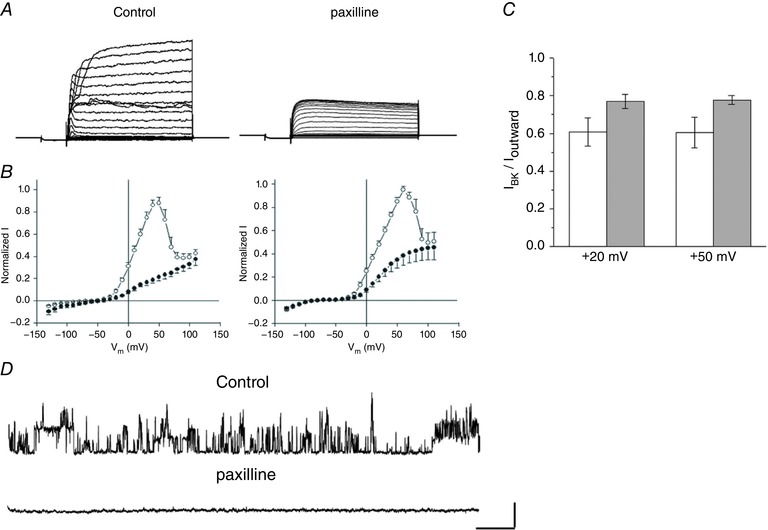
Paxilline blocks a large portion of outward currents in rats and mice. A, representative current traces before (control) and after paxilline (2.5 μm) from a rat horizontal cell. Stimulus protocols as per Fig. 1. B, summarized I–V relations showing blockade of BKCa current components in rats (left) and Cx57‐tdTomato mice (right); n = 7 cells and n = 10 cells, respectively. C, comparison of paxilline‐sensitive current between Cx57‐tdTomato mice (open bars) and rats (grey bars) at holding potentials of +20 and +50 mV. D, representative single channel traces from an outside‐out patch from a horizontal cell of a Cx57‐tdTomato mouse before (control) and during superfusion with 2.5 μm paxilline. V h = +40 mV. Scale bar: 10 pA, 100 ms.
Activation of BKCa current is closely linked to Ca2+ influx
The BKCa current in horizontal cells was mostly eliminated during superfusion with Ca2+‐free solution (Fig. 3 A–B), indicating that the activation of BKCa current is dependent on Ca2+ influx (Lee & Cui, 2010). To further evaluate the coupling between BKCa channels and voltage‐gated Cav channels, we used a voltage‐clamp protocol that isolated BKCa current and that elicited Cav channel currents with transient and steady‐state time courses (Sun et al. 2004). The voltage‐clamp protocol (Fig. 5 A and B, lower traces) has three sections: a strongly depolarized prepulse to +120 mV that activates Cav channels but is too close to E Ca to induce Ca2+ influx, an intermediate section, of increasing durations, employing a hyperpolarizing step to either −70 mV (Fig. 5 A) to induce immediate Ca2+ entry, or +20 mV (Fig. 5 B) where Cav channels remain steadily activated with sufficient driving force to allow near constant Ca2+ influx, and finally a test section where the membrane potential returns to +120 mV terminating Ca2+ entry and imposing a large outward driving force for K+. BKCa current was evoked during the final test section in Fig. 5 A by the prepulse protocol designed to generate fast but transient Ca2+ influx through Cav channels due to the large driving force during the intermediate period at −70 mV, while in Fig. 5 B the BKCa current evoked during the final test section became steady‐state in response to the prepulse that evoked a sustained Ca2+ influx at +20 mV.
Figure 5. Activation of BKCa currents closely follow Ca2+ influx dynamics in Cx57‐tdTomato mouse horizontal cells.
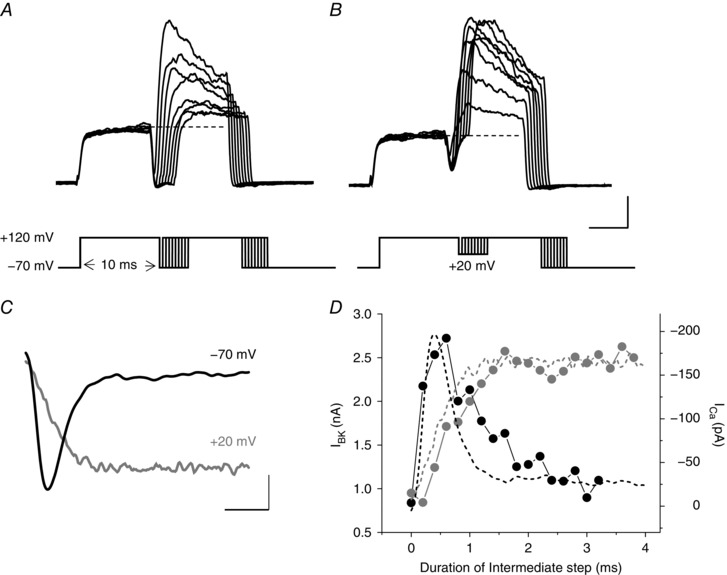
A, BKCa currents were evoked by a voltage‐clamp protocol that generates a fast and transient Ca2+ influx (via I Ca tail current elicited during an intermediate step to −70 mV), and with an increasing duration delay that allows the tail current to decay and [Ca2+]i to clear. The isolated BKCa current activates upon the second depolarization to +120 mV. B, BKCa current in response to a similar protocol, but one that evokes mostly steady‐state Ca2+ influx at +20 mV during the intermediate voltage step. C, Cav channel currents (I Ca) recorded in a separate experiment to allow comparisons of the time course of the I Ca tail current decay at −70 mV following a prepulse to +120 mV and the mostly sustained I Ca at +20 mV, again following a prepulse to +120 mV, with the dynamics of the peak BKCa currents recorded in the experiments in A and B, respectively. D, BKCa currents (lines connecting the dots) plotted against the duration of the intermediate voltage steps to −70 mV (from panel A) and to +20 mV (from panel B), are shown superimposed with the inverted Cav channel currents (dashed lines) at those respective potentials (−70 and +20 mV, in panel C). Scale bars: 0.5 nA, 5 ms in A and B; 50 pA, 1 ms in C.
The time courses of the inward Ca2+ tail current (Fig. 5 C), which is transient at −70 mV as the channels close, and the mostly sustained Ca2+ current at −20 mV, appear to drive the BKCa channel activation functions following prepulses to +120 mV in Fig. 5 A and B. The activation kinetics of outward current increments elicited by these two voltage‐clamp protocols coincide closely with the time course of Ca2+ influx (Fig. 5 D). The increment of outward current in response to these voltage protocols was blocked by either Cav or BKCa channel blockers, such as Cd2+, paxilline or iberotoxin (data not shown).
The activation of BKCa current is dependent on [Ca2+]i, which is determined by the magnitude and duration of Ca2+ influx, Ca2+ release from intracellular stores and the capacity of intrinsic Ca2+ buffering. To estimate impacts of these factors on BKCa current, we used another prepulse protocol, which first depolarized the membrane with incremental voltages and durations to load cells with Ca2+, and then tested BKCa current at +120 mV (Fig. 6 A, top). Figure 6 A show representative current traces induced by the duration‐increasing prepulse protocol. The peak currents increased with the prolongation of prepulse between 1 and 20 ms, and then became slightly depressed with further increases of the duration of depolarization (Fig. 6 C, filled symbols). In addition, the relations between peak BKCa current elicited during the step to +120 mV, as a function of the prepulse potential, are shifted to the right (Fig. 6 B). Inactivating BKCa currents in Fig. 6 A show a single exponential decay at +120 mV with time constants around 20 ms in response to the differing membrane voltages and durations of the prepulses (open symbols, Fig. 6 C). The BKCa currents from the same neuron could be blocked by Cd2+ in a dose‐dependent manner (Fig. 6 D).
Figure 6. Ca2+‐ and voltage‐dependent activation and inactivation of BKCa current in a rat horizontal cell.
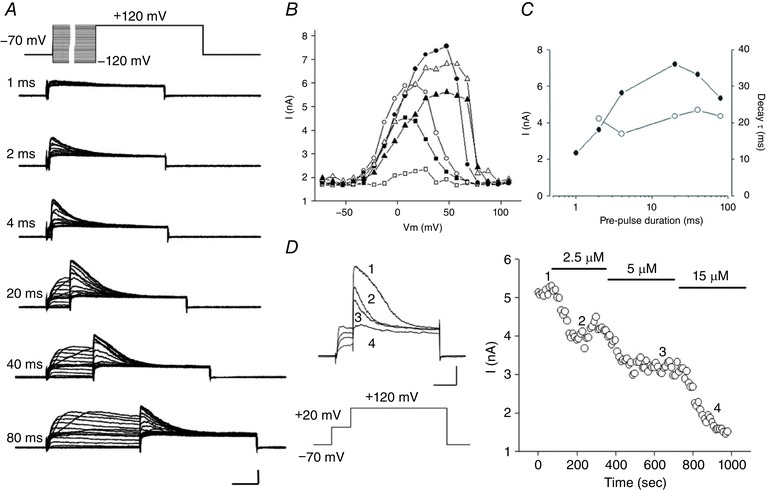
Properties of the activation/inactivation of BKCa current are revealed by varying the length of depolarizing steps to different voltages. A, representative current traces from a rat horizontal cell were obtained during a test step to +120 mV preceded by variable duration pre‐steps of incrementing voltage (top). Depolarizing pre‐step durations are labelled at the left of each set of traces. Scale bar: 2 nA, 20 ms. B, peak currents induced by test steps are plotted against the voltage of the pre‐steps having durations of 1 ms (open squares), 2 ms (filled squares), 4 ms (open circles), 20 ms (filled circles), 40 ms (open triangles) and 80 ms (filled triangles). C, peak currents (filled diamonds) and current decay time constants (open diamonds) plotted as a function of pre‐step duration on a logarithmic scale. D, Cd2+ blocks the BKCa component of outward current in a dose‐dependent manner. Left, example current traces elicited by repeating depolarizing pulse protocol (shown below currents) before (trace 1) and after superfusion of Cd2+ at concentrations of 2.5 μm (trace 2), 5 μm (trace 3) and 15 μm (trace 4). Scale bar: 1 nA, 20 ms. Right, peak current produced at +120 mV during superfusion of Cd2+. The numbers indicate the time points at which example traces shown at left were chosen.
BKCa channels co‐localize in microdomains with voltage‐gated Ca2+ channels
Ca2+ activation of K+ channels is regulated by localized cytosolic [Ca2+]i levels, which are due to influx from the extracellular space or release from intracellular stores. The previous experiments showed that BKCa channels have fast activation kinetics, coinciding with the dynamics of Ca2+ influx, suggesting that the BKCa channels are closely coupled to Ca2+ channels. The functional separation of the Cav and BKCa channels can be estimated with the use of Ca2+ buffers that have different binding kinetics (Fakler & Adelman, 2008). In Fig. 7 we examined the effects of two calcium chelators with similar binding affinity but different binding rates (k on), EGTA and BAPTA, on BKCa current. BKCa current was completely abolished by preloading the cells with BAPTA‐AM (30 μm, 1 h), while no difference from typical control BKCa currents was observed when EGTA‐AM was preloaded (30 μm, 1 h). The BK current was only tested immediately (< 5 min) following patch rupture, before wash‐out of BAPTA or EGTA from diffusion‐limited compartments could occur. The result that EGTA does not affect BKCa activation, while BAPTA does, suggests that the BKCa channels closely co‐localized with voltage‐gated Ca2+ channels (Fakler & Adelman, 2008).
Figure 7. BKCa channels co‐localize in microdomains with voltage‐gated Cav channels.
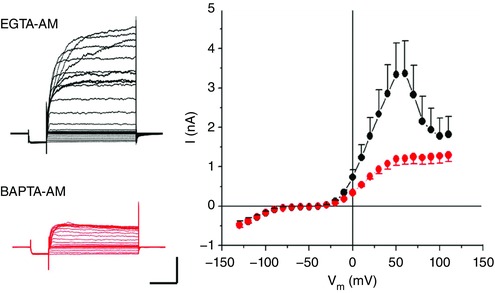
Left, representative current recordings from two horizontal cells from a Cx57‐tdTomato mouse preloaded with EGTA‐AM or BAPTA‐AM (30 μm) for 1 h. Scale bar: 1 nA, 25 ms. Right, summarized I–V relationships in mouse horizontal cells preloaded with EGTA‐AM and BAPTA‐AM; n = 7. Stimulus protocols as per Fig. 1. [Color figure can be viewed at wileyonlinelibrary.com]
Voltage and Ca2+ dependence of BKCa channel open probability
To investigate the effects of Ca2+ and voltage on single channel activity we measured open probability (P o) over a range of calcium concentrations and membrane voltages. Figure 8 shows single channel activity in inside‐out patches for the channels at four different calcium concentrations. The channels can be activated by submicromolar levels of calcium in response to strongly depolarizing voltages and the activation curves are shifted toward the left, activating at more negative potentials, with increasing calcium concentration (Fig. 8 B). A summary of the open probability (P o) of these channels, fitted with the Boltzmann equation (see Methods), gave values of half‐maximal activation (V 0.5) of +42.8 ± 2.5, −4.3 ± 3.4, −42.7 ± 2.1 and −86.1 ± 4.0 mV and slope factors (k) of 20.0 ± 2.6, 17.3 ± 2.6, 18.1 ± 2.1 and 19.0 ± 3.6 mV, in [Ca2+]i of 0. 5, 2, 20 and 100 μm, respectively (n = 5–11). The BKCa channel showed a linear current–voltage relationship between −100 and +100 mV with reversal potential near 0 mV in symmetrical 150 mm K+ (Fig. 8 C). The values of single channel conductance calculated by linearly fitting I–V curves from each individual patch were in the range of 202–279 pS (244 ± 11 pS, n = 17).
Figure 8. Ca2+‐ and voltage‐dependent activation of single BKCa channels.
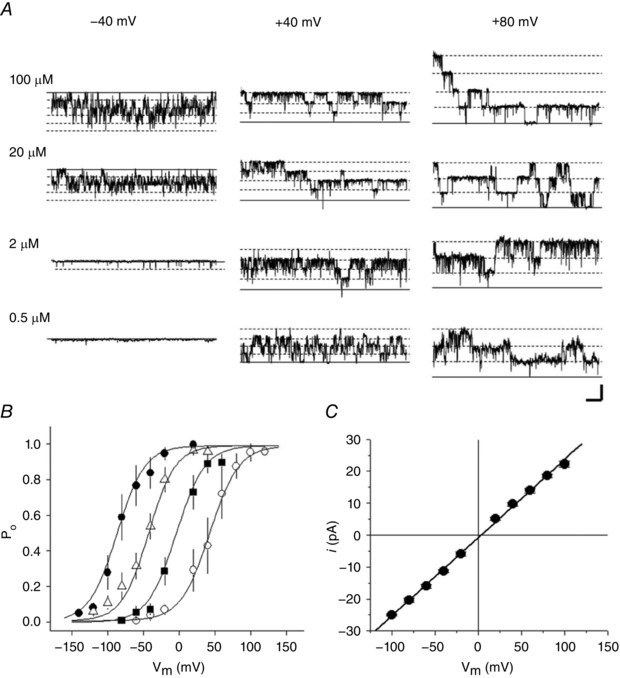
Single channel currents were recorded from an inside‐out patch excised in symmetrical 150 mm K+ solutions from a horizontal cell from a Cx57‐tdTomato mouse retina. A, sample current traces at three holding potentials (columns labelled −40, +40 and +80 mV) and four nominal [Ca2+]i values (rows labelled 100, 20, 2 and 0.5 μm). Continuous and dashed lines indicate channel closed or open states, respectively. B, open probability (P o) of BKCa channels is plotted against holding potentials in [Ca2+] of 0.5 μm (open circles), 2 μm (squares), 20 μm (triangles) and 100 μm (filled circles). The smooth lines were generated by fitting the data points (symbols) with the Boltzmann equation (see text). Scale bar: 20 pA, 100 ms. C, single channel I–V curve fitted with line having slope conductance of 234 pS.
The fast inactivation kinetics seen in whole‐cell currents suggests that these BKCa channels might have β subunits, since some β subunits produce fast inactivation in BKCa channels (Hicks & Marrion, 1998). To test for a role of this auxiliary subunit, we applied trypsin to the cytosolic membrane of mouse horizontal cells in order to determine if the time constant of inactivation would be lengthened by proteolytic treatment. Ensemble average currents (Fig. 9 A), constructed from single channel currents (Fig. 9 B), elicited by a depolarizing voltage step showed a near complete elimination of inactivation following superfusion with 0.5 mg ml−1 trypsin in inside‐out patches (n = 3).
Figure 9. Trypsin eliminates inactivation of BKCa channels.
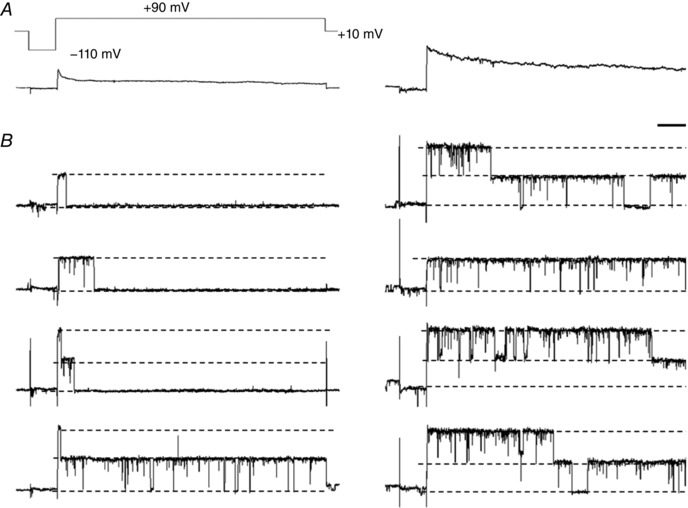
A, ensemble average currents made from single channel currents elicited by a voltage step with a hyperpolarizing prepulse (top) before (left) and after (right) superfusion of 0.5 mg ml−1 trypsin in an inside‐out patch of a horizontal cell from a Cx57‐tdTomato mouse. B, representative single channel traces used for ensemble averages (A). The numbers of single channel traces for ensemble averages were 150 and 100 in the absence and presence of trypsin, respectively. Scale bar: 10 pA, 50 ms.
Contribution of BKCa channel to horizontal cell membrane potential excursions
In horizontal cells recorded with normal cytosolic [Ca2+]i levels, BKCa channels begin their activation positive to −30 mV, suggesting that they will have strongly rectifying actions on membrane potentials that exceed this value. We tested membrane voltage behaviour in current‐clamped horizontal cells before and after applying a BKCa channel blocker, as well as a BKCa channel activator, to characterize the role played by BKCa channels in membrane potential oscillations. The overall, averaged effects of BKCa channel activity were apparent well below this relatively depolarized voltage range. We first tested the BKCa channel blocker paxilline on the responses of horizontal cells to hyperpolarizing and depolarizing current injections. The effects of paxilline were pronounced in many horizontal cells, allowing the cell to produce oscillatory, transient spikelets in response to depolarizing current injection (Fig. 10 A). The averaged horizontal cell voltage–current relations (Fig. 10 C) showed that paxilline increased membrane potential depolarization in response to current injection compared to control. The effects of NS1619 were opposite to these, suppressing the amplitude and the modest oscillatory activity of this cell in response to depolarizing current injection (Fig. 10 B). Averaged horizontal cell voltage–current relations in NS1619 (Fig. 10 D) showed reduced membrane potential depolarization during the most depolarizing current injections compared to control.
Figure 10. Effect of BKCa channel block and enhancement on voltage changes of horizontal cells in response to current injection.
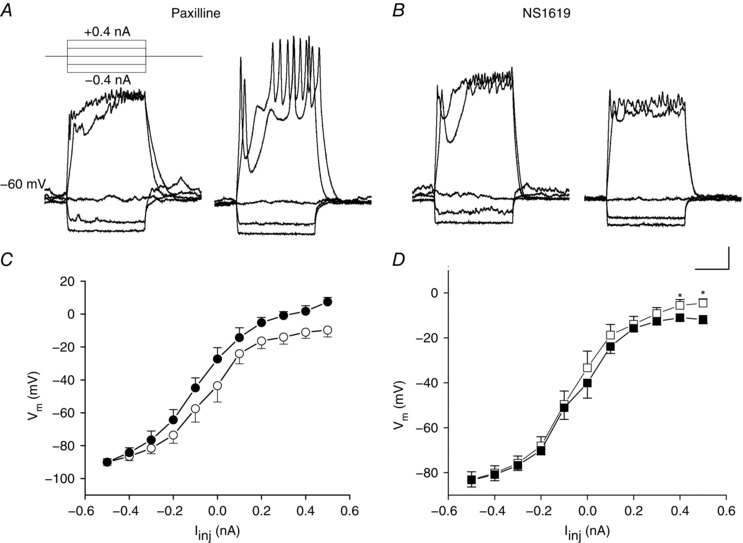
A, horizontal cells from Cx57‐tdTomato mice were studied with whole‐cell patch clamp in current clamp mode. Membrane potential responses to current steps (inset) were obtained before (left) and after (right) application of the BKCa channel blocker paxilline (2.5 μm). B, voltage changes before (left) and after (right) application of the BKCa channel activator NS1619 (30 μm). Scale bar: 10 mV, 20 ms. C, summarized voltage–current relations before (open circles) and after paxilline (filled circles; n = 8, P < 0.001, ANOVA, values >0.0 nA). D, summarized voltage–current relations before (open squares) and after (filled squares) application of NS1619. (n = 14, * P < 0.05, paired t test).
We also examined the effects of paxilline and NS1619 on resting membrane potentials of horizontal cells in Cx57‐tdTomato mice. Figure 11 A shows that paxilline increased depolarizing membrane potential excursions, producing noisy oscillations of increasing frequency, while NS1619 inhibited these noisy oscillations (Fig. 11 B). The summary in Fig. 11 C shows that paxilline increased the average membrane potential by about 8 mV and NS1619 reduced the average membrane potential by about the same amount (Fig. 11 D). Due to the strong electrical coupling between horizontal cells (Shelley et al. 2006), large spontaneous depolarizations, seen here in isolated horizontal cells, are probably not present in vivo. Activity of BKCa channels is likely to set an upper limit to physiologically attainable voltages and may influence response kinetics, especially broad field light fluctuations, but it is unlikely that the large and rapid voltage fluctuations seen in Fig. 11 are observed in the intact retina.
Figure 11. BKCa channel influence on horizontal cell membrane potential oscillations.
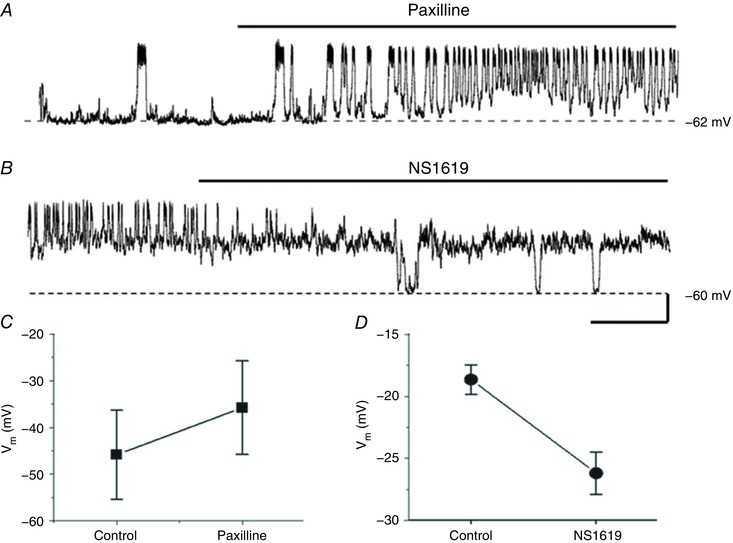
A and B, examples of oscillatory resting membrane potential activity in response to bath application of the BKCa channel blocker paxilline (2.5 μm; A), and the BKCa channel activator NS1619 (30 μm; B), in the same isolated, mouse Cx57‐tdTomato horizontal cell. Scale bar: 20 mV, 1 min. C, summarized average membrane potential changes before and after 2.5 μm paxilline (n = 6, P < 0.05, paired t test). D, summarized average membrane potential changes before and after 30 μm NS1619 (n = 6, P < 0.01, paired t test).
Discussion
The analysis presented here leads to the conclusion that the major part of the outward current in horizontal cells, elicited at depolarized membrane potentials positive to −30 mV, is carried through BKCa channels in rat and mouse retina. Our identification of BKCa channels is based on assessment of the biophysical and pharmacological properties of the single channel and whole‐cell currents observed (Lee & Cui, 2010). The response‐shaping influences of this channel type on horizontal cell membrane potential appear to potently regulate cell behaviour to depolarizing stimuli.
Horizontal cell outward currents meet key criteria that define BKCa currents
The properties of currents we characterized in horizontal cells match the two criteria that typically define the key features of BKCa channel currents. First, analysis of the whole‐cell current shows a bell‐shaped current–voltage relation, defined under voltage clamp, which arises from the channels’ dependence on both voltage and cytosolic [Ca2+] (Lee & Cui, 2010). Outward current in horizontal cells under normal [Ca2+]i levels begins activation near −30 mV and then peaks and falls off in the current–voltage relation at potentials positive to +50 mV, observations typical of BKCa channels arising from the lessening driving force on Ca2+, which leads to reduced [Ca2+]i levels as membrane potential mounts. Second, the BKCa channel is characterized by its large single channel conductance, which falls in a range, depending on ionic conditions, between 100 and 300 pS (Lee & Cui, 2010). In horizontal cells recorded with physiological concentrations of K+, we found the single channel conductance to be just over 100 pS, while under symmetrical 150 mm K+, we found the single channel conductance to be about 250 pS. We showed these characteristic features of the BKCa channels in parallel by comparing the activation range in both whole‐cell and single channel recording configurations. We note that a current having an I–V relation with a shape very similar to what we observed here was previously reported in mouse horizontal cells and considered to be an ether‐à‐go‐go‐related gene (erg1) channel current (Feigenspan et al. 2009). That study did not report the properties and calcium dependence of the underlying single channels. The value of their single channel conductance distinguishes BKCa channels from erg channels effectively as this number is 10–30 times greater in BKCa than erg channels (Zou et al. 1997; Abbott et al. 1999). Our values of 100 pS in physiological conditions and 250 pS in symmetrical K+ define the currents we recorded as BKCa channel currents. A later immunohistochemical study showed erg channels were not expressed in mouse horizontal cells (Cordeiro et al. 2011).
Pharmacological sensitivity of horizontal cell BKCa channels
Pharmacological analysis further confirmed the identity of the horizontal cell outward current with a trio of well‐established agents, these being the BKCa channel activator NS1619 and the BKCa channel blockers iberiotoxin and paxilline, with blockade by the latter shown in both whole‐cell and single channel recordings. Owing to their potent effects on BKCa currents under voltage clamp, paxilline and NS1619 were used in further experiments under current clamp to show the actions of reduced or enhanced BKCa conductance on the depolarized membrane potential fluctuations in horizontal cells. The effects of paxilline and NS1619 shown under current clamp (Fig. 11) may have been influenced by the different membrane potentials at which the cell rested. That is, the potency of paxilline action may be enhanced at negative potentials and that of NS1619 enhanced at positive potentials. The results show that BKCa channel activation or inactivation may contribute to the characteristic membrane potential fluctuations seen in these isolated horizontal cells.
Ca2+ dependence of BKCa current activation
Our studies confirmed the Ca2+ sensitivity of whole‐cell BKCa current activation, showing that in Ca2+‐free solutions the current was absent, while at the single channel level, we defined the sensitivity of channel open probability over a broad range of cytosolic membrane [Ca2+] levels in excised, inside‐out patch clamp recordings. The sensitivity of BKCa channels to Ca2+ influx is generally considered to result from a relatively low Ca2+ affinity of the channel, a high degree of Ca2+ cooperativity and a close co‐localization with Ca2+ channels (Art et al. 1995; Sakaba et al. 1997; Yazejian et al. 2000; Llobet et al. 2003; Sun et al. 2004; Palmer, 2006). The BKCa current recorded in horizontal cells exhibited fast activation kinetics that coincided with the dynamics of Ca2+ influx. In two separate tests (Fig. 5), the time course of Ca2+ influx defined the degree of activation of BKCa current. (1) When the membrane was stepped from a potential of +120 mV, at which Ca2+ channels are activated but due to the low driving force, no Ca2+ influx occurs, to −70 mV, a potential at which Cav channels rapidly inactivate under a strong driving force for Ca2+ that produces Ca2+ influx (heavy, transient line in Fig. 5 C), this influx activates BKCa current. However, there is almost no driving force on K+, so the membrane is stepped back up to +120 mV after a delay to allow some Ca2+ influx. Thus at +120 mV, BKCa current is visible, and we compared its peak amplitude as a function of the duration of the step to −70 mV (dark dots in Fig. 5 D), comparing this to the deactivating Ca2+ tail current measured at −70 mV (heavy dashed line in Fig. 5 D) and found that the superposition was strong. (2) When we stepped the cell to −20 mV, instead of −70 mV during the midway step (Fig. 5 B), and obtained mostly sustained Ca2+ tail currents (Fig. 5 C, light line), the comparison of this with the subsequently activated BKCa current at +120 mV again showed near perfect superposition (Fig. 5 D, light lines and dots). These results show the tight relationship between Ca2+ influx and BKCa current.
When we assessed the relationships between membrane potential and activation of BKCa current at increasingly depolarized membrane voltage steps, a high degree of inactivation following activation became evident. Testing BKCa current kinetics at +120 mV, following increasingly depolarized prepulses, we found that while BKCa current amplitude reached a peak at +50 mV following prepulse steps that lasted at least 20 ms, longer prepulses led to less BKCa currents, due to inactivation. We noted the inactivation time constant at +120 mV, which represents the clearance of Ca2+ at that potential since there is no further Ca2+ influx, was nearly constant at about 20 ms. These results affirm that [Ca2+]i is the strongest driver of BKCa channel activation, and that Ca2+ influx is by far the greatest influence on activation followed by intrinsic Ca2+ buffering.
Finally, the rapid inactivation kinetics of horizontal cell BKCa channels may imply conjugation with β subunits. The four BKCa β subunits, which share the same structure and are composed of two transmembrane segments, confer different properties to channel gating, as follows: β1 increases the Ca2+ sensitivity of the channel, slows activation and deactivation kinetics and shifts the activation curve up to 80 mV negative; β2 also increases Ca2+ sensitivity but promotes inactivation; β3, composed of four subunits arising from alternative splicing, produces increased channel inactivation with β3a, b and c, but not β3d; and β4 dramatically slows BK channel activation (Torres et al. 2014). BKCa channel β subunits are highly sensitive to trypsin and when present can remove inactivation of the BKCa channel (Hicks & Marrion, 1998; Marrion & Tavalin, 1998). When we recorded from inside‐out patches from mouse horizontal cells (Fig. 9), inactivation of BKCa channel activity was nearly fully eliminated following application of trypsin, supporting the possibility that pore‐forming BKCa channel α subunits are co‐expressed with β2 or β3 subunits due to requirements for fast channel inactivation.
BKCa channels are situated in close proximity to Cav channels
Mammalian horizontal cells express an array of voltage‐gated Cav channels, including L‐, N‐ and P/Q‐types, which supply Ca2+ influx at potentials of −40 mV and above (Ueda et al. 1992; Löhrke & Hofmann, 1994; Schubert et al. 2006; Witkovsky et al. 2006; Liu et al. 2013; Liu et al. 2016). In Fig. 8, complete block of BKCa currents occurred with 15 μm Cd2+, a potent Cav channel blocker and the voltage dependence of activation of single channel currents increases with Ca2+ concentrations increasing to 0.5, 2, 20 and 100 μm. The activation curve measured with 20 μm Ca2+ seems to provide the closest agreement to the excised, outside‐out patch, single channel recording of Fig. 2, which shows maximal activation occurring by −25 mV. It has been estimated that to activate BKCa channels, local intracellular Ca2+ concentrations of about 10 μm are required (Magleby, 2003). Ca2+ concentrations of such a high level are only achieved in the microdomains around open Ca2+ channels (Heidelberger et al. 1994; Matthews, 1996; Beaumont et al. 2005; Schneggenburger & Neher, 2005). BKCa channel activation requires, therefore, very tight linkage to activation of Cav channel currents, and not spatially broad intracellular Ca2+ concentration (Yazejian et al. 2000). BKCa channels in horizontal cells appear to tightly co‐localize in submicrodomains with voltage‐gated Cav channels. Our results showed a failure of activation of BKCa channels when incubated with BAPTA‐AM whereas the same treatment with EGTA‐AM produced large and normal BKCa channel activation. Since the binding affinities of BAPTA and EGTA for Ca2+ are similar but BAPTA binds Ca2+ 40 times faster, BAPTA affects Ca2+ signalling to effectors only if the diffusion distance for Ca2+ is on the order of 10–20 nm, and EGTA does not bind Ca2+ fast enough to alter Ca2+ signalling over this distance (Williams et al. 2012). The fact that BAPTA‐AM substantially reduced the activation of BKCa channels, whereas EGTA‐AM permitted their robust activation, indicates that BKCa channel activation occurs via closely apposed Cav channels and not by changing bulk [Ca2+]i.
Regulation of horizontal cell membrane potential
Horizontal cell depolarization inhibits photoreceptor neurotransmitter release (reviewed in Thoreson & Mangel, 2012). Inhibition of photoreceptors increases with horizontal cell depolarization so one question is, how positive can horizontal cells go? We show here that BKCa channels effectively limit isolated horizontal cell depolarization to a value in the region of −25 mV, although intermittent spikelets exceed this value. In our experiments with isolated horizontal cells, there is no feedback loop between horizontal cells and photoreceptors. Were the loop intact, then horizontal cell depolarization would lead to photoreceptor Cav channel inhibition and, therefore, reduced release of glutamate. As a consequence, horizontal cells would not be depolarized as much. A dark resting value of −33 mV was noted in mouse horizontal cells in retinal flatmounts (Shelley et al. 2006), showing a greater regulation of depolarization present in the intact network.
Horizontal cells may also take part in the temporal filtering of signals passing from photoreceptors to bipolar cells. We isolated the horizontal cell membrane from the actions of photoreceptor glutamate release in our current clamp experiments by recording from horizontal cells dissociated from the retina. We observed membrane bistability reminiscent of much earlier observations in non‐mammalian vertebrate retinas (Shingai & Christensen, 1986). In addition to the horizontal cell having bistable states of approx. −60 and −25 mV, when in the depolarized state, small depolarizing spikelets occurred, the frequency of which depend on BKCa channel availability. This suggests that, as in some cochlear hair cells where they have such a function (Pyott & Duncan, 2016), BKCa channels could play a role in tuning horizontal cell frequency response characteristics, with concomitant effects on the bandpass properties of photoreceptor to bipolar cell synaptic transfer. ERGs recorded in BKCa knock‐out mice showed reduced b‐waves at mesopic intensities, but not low scotopic or photopic levels (Tanimoto et al. 2012). This feature was previously attributed to BKCa channel‐regulated A17 amacrine cell feedback onto rod bipolar cells (Grimes et al. 2009). In that report, BKCa channels on A17 dendrites activated by excitatory synaptic input from rod bipolar cells appeared to reduce reciprocal inhibitory GABAergic feedback from A17s to the bipolar cell axon terminals. Thus BKCa channel activity can modulate the dynamic range of bipolar cell responses. Our work now defines the presence of BKCa channels in horizontal cells and raises the possibility that these channels in this cell type could also contribute to b‐wave modulation, either through the feedback pathway to photoreceptors or by direct feed forward to bipolar cells (Thoreson & Mangel, 2012).
Additional information
Competing interests
None declared.
Author contributions
X.S.: conception and design; collection and assembly of data; data analysis and interpretation. A.H.: conception and design; financial support; provision of study materials or patients. N.B.: conception and design; financial support; administrative support; provision of study materials or patients. S.B.: conception and design; financial support; data analysis and interpretation; manuscript writing. The work was performed at UCLA and Dalhousie University. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Support for this work was provided by grants from the National Eye Institute (EY15573 to N.C.B.), a UCLA Oppenheimer Seed Grant (A.A.H., N.C.B.), the Plum Foundation (S.B. and N.C.B.), the Canadian Institutes of Health Research (MOP‐10968 to S.B.), and the National Science and Engineering Research Council (Discovery Award 194640 to S.B.). N.C.B. is a Veterans Administration Career Scientist.
N. C. Brecha and S. Barnes are co‐senior authors.
Linked articles This article is highlighted by a Perspective by Thoreson. To read this Perspective, visit https://doi.org/10.1113/JP274419.
References
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT & Goldstein SA (1999). MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97, 175–187. [DOI] [PubMed] [Google Scholar]
- Art JJ, Wu YC & Fettiplace R (1995). The calcium‐activated potassium channels of turtle hair cells. J Gen Physiol 105, 49–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, Llobet A & Lagnado L (2005). Expansion of calcium microdomains regulates fast exocytosis at a ribbon synapse. Proc Natl Acad Sci USA 102, 10700–10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia S, Garcia ML & Latorre R (1992). Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+‐activated K+ channel. Biophys J 63, 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro S, Guseva D, Wulfsen I & Bauer CK (2011). Expression pattern of Kv11 (ether à‐go‐go‐related gene; erg) K+ channels in the mouse retina. PLoS One 6, e29490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B & Adelman JP (2008). Control of KCa channels by calcium nano/microdomains. Neuron 59, 873–881. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Trumpler J, Dirks P & Weiler R (2009). Ether‐à‐gogo‐related gene (erg1) potassium channels shape the dark response of horizontal cells in the mammalian retina. Pflugers Arch 458, 359–377. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chavez AE & Diamond JS (2009). BK channels modulate pre‐ and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci 12, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E & Matthews G (1994). Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371, 513–515. [DOI] [PubMed] [Google Scholar]
- Hicks GA & Marrion NV (1998). Ca2+‐dependent inactivation of large conductance Ca2+‐activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J Physiol 508, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Liu X, Boulter J, Grove J, Perez de Sevilla Muller L, Barnes S & Brecha NC (2016). Targeted deletion of vesicular GABA transporter from retinal horizontal cells eliminates feedback modulation of photoreceptor calcium channels. eNeuro 3, ENEURO.0148‐15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee US & Cui J (2010). BK channel activation: structural and functional insights. Trends Neurosci 33, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Grove JC, Hirano AA, Brecha NC & Barnes S (2016). Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. J Neurophysiol 116, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC & Barnes S (2013). Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA‐ and pH‐sensitive mechanism. J Physiol 591, 3309–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet A, Cooke A & Lagnado L (2003). Exocytosis at the ribbon synapse of retinal bipolar cells studied in patches of presynaptic membrane. J Neurosci 23, 2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhrke S & Hofmann HD (1994). Voltage‐gated currents of rabbit A‐ and B‐type horizontal cells in retinal monolayer cultures. Vis Neurosci 11, 369–378. [DOI] [PubMed] [Google Scholar]
- Magleby KL (2003). Gating mechanism of BK (Slo1) channels: so near, yet so far. J Gen Physiol 121, 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV & Tavalin SJ (1998). Selective activation of Ca2+‐activated K+ channels by co‐localized Ca2+ channels in hippocampal neurons. Nature 395, 900–905. [DOI] [PubMed] [Google Scholar]
- Matthews G (1996). Neurotransmitter release. Annu Rev Neurosci 19, 219–233. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Munch E, Moldt P & Drejer J (1994). Selective activation of Ca2+‐dependent K+ channels by novel benzimidazolone. Eur J Pharmacol 251, 53–59. [DOI] [PubMed] [Google Scholar]
- Palmer MJ (2006). Modulation of Ca2+‐activated K+ currents and Ca2+‐dependent action potentials by exocytosis in goldfish bipolar cell terminals. J Physiol 572, 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L & Gonzalez‐Soriano J (1994). Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci 11, 501–517. [DOI] [PubMed] [Google Scholar]
- Pyott SJ & Duncan RK (2016). BK channels in the vertebrate inner ear. Int Rev Neurobiol 128, 369–399. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Ishikane H & Tachibana M (1997). Ca2+‐activated K+ current at presynaptic terminals of goldfish retinal bipolar cells. Neurosci Res 27, 219–228. [DOI] [PubMed] [Google Scholar]
- Sanchez M & McManus OB (1996). Paxilline inhibition of the alpha‐subunit of the high‐conductance calcium‐activated potassium channel. Neuropharmacology 35, 963–968. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R & Neher E (2005). Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol 15, 266–274. [DOI] [PubMed] [Google Scholar]
- Schubert T, Weiler R & Feigenspan A (2006). Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J Neurophysiol 96, 1278–1292. [DOI] [PubMed] [Google Scholar]
- Shelley J, Dedek K, Schubert T, Feigenspan A, Schultz K, Hombach S, Willecke K & Weiler R (2006). Horizontal cell receptive fields are reduced in connexin57‐deficient mice. Eur J Neurosci 23, 3176–3186. [DOI] [PubMed] [Google Scholar]
- Shingai R & Christensen BN (1986). Excitable properties and voltage‐sensitive ion conductances of horizontal cells isolated from catfish (Ictalurus punctatus) retina. J Neurophysiol 56, 32–49. [DOI] [PubMed] [Google Scholar]
- Sun XP, Schlichter LC & Stanley EF (1999). Single‐channel properties of BK‐type calcium‐activated potassium channels at a cholinergic presynaptic nerve terminal. J Physiol 518, 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XP, Yazejian B & Grinnell AD (2004). Electrophysiological properties of BK channels in Xenopus motor nerve terminals. J Physiol 557, 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto N, Sothilingam V, Euler T, Ruth P, Seeliger MW & Schubert T (2012). BK channels mediate pathway‐specific modulation of visual signals in the in vivo mouse retina. J Neurosci 32, 4861–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB & Mangel SC (2012). Lateral interactions in the outer retina. Prog Retin Eye Res 31, 407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres YP, Granados ST & Latorre R (2014). Pharmacological consequences of the coexpression of BK channel α and auxiliary β subunits. Front Physiol 5, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kaneko A & Kaneda M (1992). Voltage‐dependent ionic currents in solitary horizontal cells isolated from cat retina. J Neurophysiol 68, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Williams C, Chen W, Lee CH, Yaeger D, Vyleta NP & Smith SM (2012). Coactivation of multiple tightly coupled calcium channels triggers spontaneous release of GABA. Nat Neurosci 15, 1195–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Shen C & McRory J (2006). Differential distribution of voltage‐gated calcium channels in dopaminergic neurons of the rat retina. J Comp Neurol 497, 384–396. [DOI] [PubMed] [Google Scholar]
- Wrzosek A (2014). The potassium channel opener NS1619 modulates calcium homeostasis in muscle cells by inhibiting SERCA. Cell Calcium 56, 14–24. [DOI] [PubMed] [Google Scholar]
- Yazejian B, Sun XP & Grinnell AD (2000). Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+‐activated K+ channels. Nat Neurosci 3, 566–571. [DOI] [PubMed] [Google Scholar]
- Zou A, Curran ME, Keating MT & Sanguinetti MC (1997). Single HERG delayed rectifier K+ channels expressed in Xenopus oocytes. Am J Physiol Heart Circ Physiol 272, H1309–H1314. [DOI] [PubMed] [Google Scholar]


