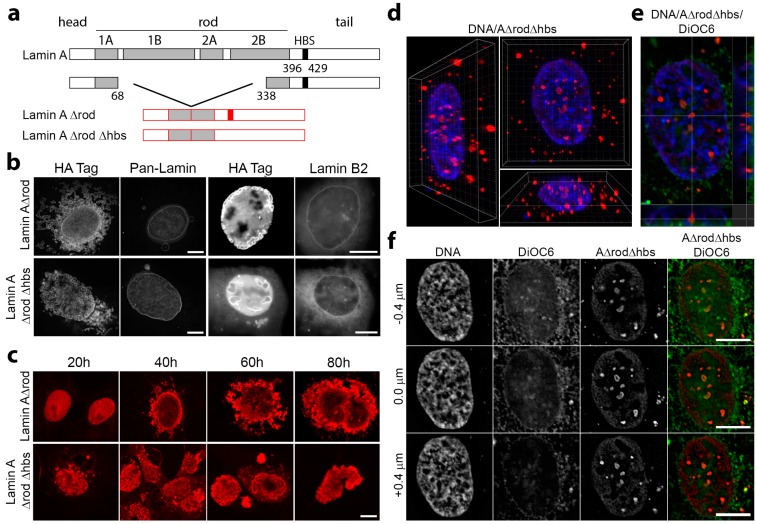
Figure 5.
A lamin A mutant generated to increase the density of histone-binding sites assembles intranuclear structures independent of the peripheral lamina. (a) The lamin A/C rod domain is similar in mass to the globular tail domain of lamin A that contains the mapped lamin A/C histone-binding site. Thus, in A∆rod, most of the rod was deleted to roughly double the mass density of histone-binding sites. As a negative control, A∆rod∆hbs also deleted the major mapped histone-binding site. (b) When A∆rod was expressed in U2OS cells, it formed circular structures both in the nucleus and in the cytoplasm. In most cells, these structures neither integrated with nor perturbed the peripheral lamin structural network, as indicated by co-staining for the lamin A∆rod mutants and for endogenous lamins with a pan-lamin antibody or an antibody to the similar region in lamin B2. (c) Expressing the lamin A constructs in COS-7 cells to match the earlier B1∆rod experiments, some A∆rod and A∆rod∆hbs structures were formed by 20 h post-transfection and most by 40 h. (d,e) 3D reconstructions of cells, produced from serial z-sections (0.2-μm step size) after deconvolution using Imaris 8 software revealed that the A∆ structures tend to be completely internal without connecting to the membrane. (f) Co-staining with the membrane dye DiOC6 further confirmed that the internal structures were free from membrane, and thus, results could not be attributed to nuclear membrane proteins. All scale bars, 10 µm.