Figure 1.
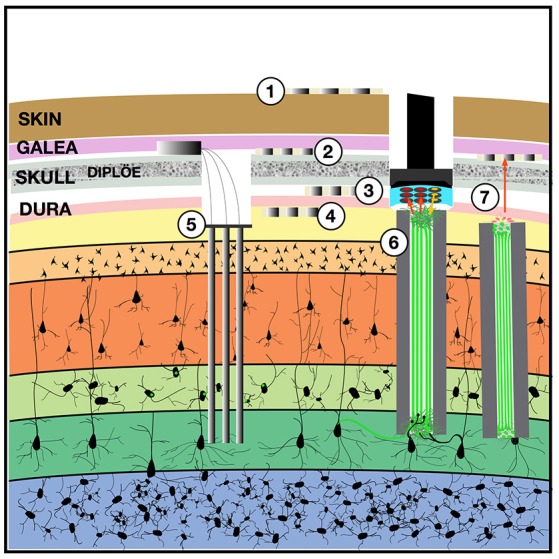
Implantable devices to chronically stimulate and record from the brain. Both low-impedance macro-electrodes and higher-impedance more densely packed electrodes can be used for electroencephalography (EEG) with contacts placed on the (1) scalp, (2) implanted in the subgaleal space without breaching the skull, (3) in the epidural space, or (4) in the subdural space where they may also be termed the electrocorticogram (ECoG) or micro-ECoG (Yu et al., 2016). These electrodes can capture local field potentials and can also be used to pass electrical current. To record ensembles of single units, (5) multi-electrode arrays can be implanted into the cortex, with or without integrated optical fibers. One solution to the apparent biological instability of rigid microelectrodes chronically implanted into the parenchyma, is to create a (6) “living electrode” comprising autologous neurons seeded within an agarose minicolumn that itself can be stereotactically implanted (Struzyna et al., 2015; Adewole et al., 2016). This three-dimensional living construct can both send axons to stimulate surrounding cortex and receive synapses to capture local activity and transmit this to an aggregate on the cortical surface where a planar optoelectronic array can reciprocally relay recordings and stimulation triggers with an external computer. The externalized aspect of the living electrode (7) could be capped with myocytes to achieve biopotential amplification such that signals could be captured by a subgaleal grid. That grid could in turn wirelessly broadcast signals to external computers. The subgaleal grid could also achieve stimulation of the brain, to provide input and feedback, by transmitting microcurrents to ephaptically modulate the living electrode cap in a manner analogous to the ampullae of Lorenzini in chondrichthyes, chondrostei and teleost fish and monotreme mammalian electroception structures.
