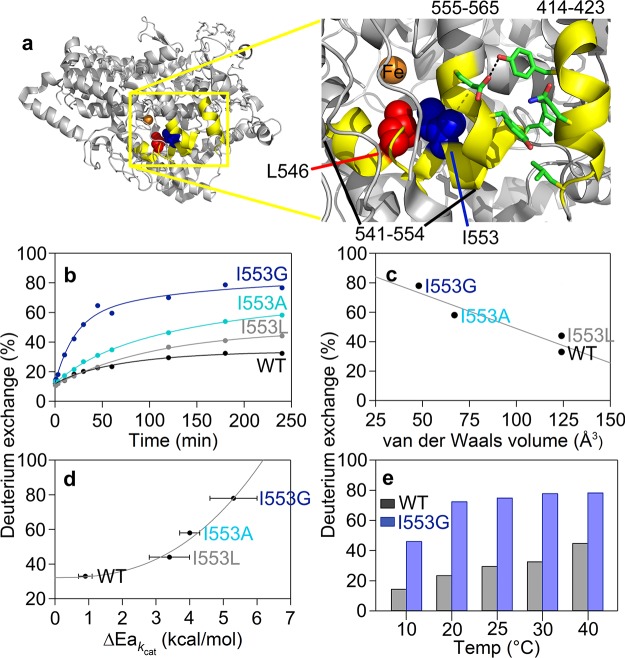
Figure 2.
Active site (proximal) peptides (in yellow) that exhibit mutation-induced increases in the percent of deuterium exchange at 4 h. (a) The highlighted region of the protein is shown in the yellow box. The key active site residues, undergoing mutation in this study, are shown as space filling models: L546 (red) and I553 (blue). The residues participating in noncovalent interactions between 555–565 and 414–423 are shown as sticks. (b) HDX traces for the representative peptide, 555–565, at 30 °C with three I553 variants. (c) Percent exchange at 4 h from panel b is plotted versus the van der Waals volume of the 553 side chain. (d) Percent exchange at 4 h from panel b is plotted as a correlation to the temperature dependence of the catalytic Dkcat (ΔEa; Table S3). (e) Differences in percent exchange for 555–565 between WT and I553G at 4 h are observed at all temperatures.