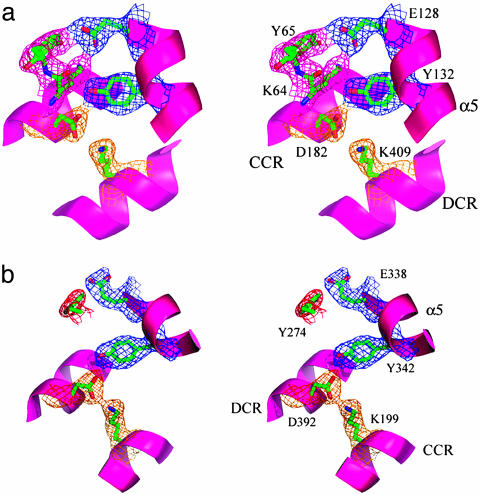
Fig. 5.
Stereo presentations of the TRD's interaction with the CRs. The longest helix, α5, in the TRDs interacts with CR helices by providing hydrophobic residues at the ends of the CRs as well as hydrophilic residues toward the middle of CR helices that were displayed with 2 Fo - Fc density contoured at 1.5 σ. The figures were prepared by using pymol (www.pymol.org). (a) Lys-64 in TRD1 is located at the interface with the CRs and has a well defined electron density. However, it has a distorted geometry. (b) The interaction of TRD2 with CRs is simplified compared with that of TRD1 probably because of the loss of a hydrogen bond donor corresponding to Lys-64 in TRD1.