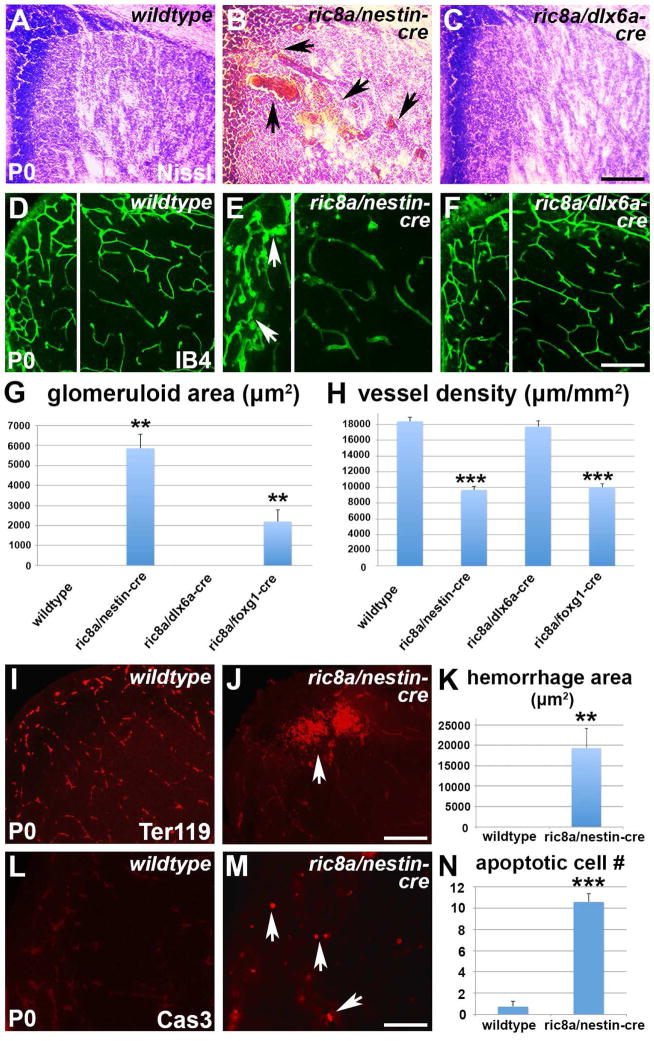
Figure 1. Ric8a is required in neural progenitors to regulate vessel development in the LGE.
See also Figure S1.
(A–C) Nissl staining of P0 striata from wildtype (A), ric8a; nestin-cre (B) and ric8a; dlx6a-cre (C) mutant animals. Note hemorrhages in B (arrows).
(D–F) Isolectin B4 (IB4) labeling of vessels in P0 striata of wildtype (D), ric8a; nestin-cre (E) and ric8a; dlx6a-cre (F) animals. Note glomeruloid structures in E (arrows).
(G) Quantification of areas with glomeruloid structures in P0 ventricular zones (VZs) shows significant increases in nestin-cre and foxg1-cre, but not dlx6a-cre mutants. **, p < 0.01; n = 5.
(H) Quantification of vessel density in P0 striata shows severe reductions in nestin-cre and foxg1-cre, but not dlx6a-cre mutants. ***, p < 0.001; n = 7.
(I–K) Hemorrhage in ric8a; nestin-cre mutant striata. Anti-Ter119 labeling of red blood cells (I–J) shows large accumulations outside vessels in mutants (arrows in L). (K) Quantification of hemorrhage areas. **, p < 0.01; n = 6.
(L–N) Cell death in ric8a; nestin-cre mutant striata. Anti-cleaved caspase3 labeling (L–M) shows increased numbers of apoptotic cells in mutants (arrows in M). (N) Quantification of apoptotic cell numbers per field. ***, p < 0.001; n = 5.
Scale bars: 200 μm for A–F, I, J, L, and M.