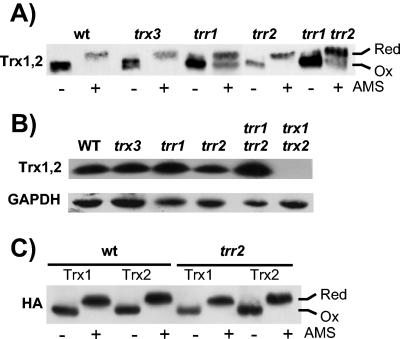
FIG. 4.
Redox state of mitochondrial thioredoxin system mutants. (A) Redox state of cytoplasmic thioredoxins. The indicated strains were grown to exponential phase (A600 = 1.0) in SD medium. Proteins were precipitated with TCA, and free thiols were modified by reaction with AMS. Samples were separated using SDS-18% PAGE, and cytoplasmic thioredoxins (Trx1 and Trx2) were detected by Western blot analysis with rabbit anti-yeast thioredoxin antibody (Trx1,2). Fully oxidized and fully reduced proteins are indicated. (B) Western blot analysis of thioredoxin protein levels. Thioredoxin protein concentrations were measured in wild-type and trx3, trr1, trr2, trr1 trr2, and trx1 trx2 mutant strains by Western blot analysis with antibodies specific for thioredoxins (Trx1,2) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (C) Trx1 and Trx2 were epitope tagged with a hemagglutinin (HA) tag to determine the redox state of each cytoplasmic thioredoxin isoform. Trx1 and Trx2 are both present in the fully reduced form in a wild-type strain and a strain lacking mitochondrial Trr2.