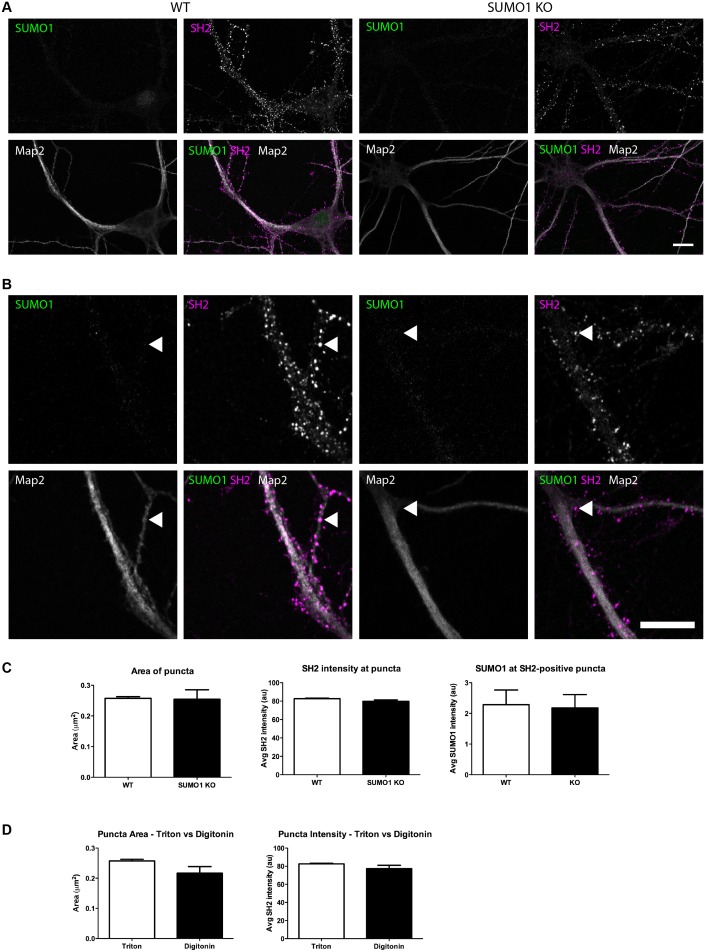
Figure 15. Quantification of anti-SUMO1 immunolabelling shows that SUMO1 is not specifically detected at postsynaptic sites in Triton X-100-permeabilised neurons.
(A) Primary hippocampal neurons from WT and SUMO1 KO littermates were fixed, then triton X-100-permeabilised, and immunolabelled with antibodies against SUMO1 (green), shank2 (purple), and Map2 (white). A region of interest (neuron) was identified, automatically divided into a grid composed of 6–12 fields of view, and z-stacks were taken for each field. Fields of view were 'stitched' back together to form overview images. (B) Inset regions from (A) are shown in greater detail. (C) Shank2-positive puncta were automatically segmented and the area (left panel) and fluorescence intensity (centre panel) were calculated in shank2-positive regions of interest (puncta). The intensity of anti-SUMO1 immunolabelling was also quantified at shank2-positive puncta (right panel). No significant difference between WT and SUMO1 KO neurons was observed for anti-SUMO1 intensity at shank2-positive puncta. These data indicate that anti-SUMO1 staining at synaptic sites is not specific for SUMO1. (D) Graphs comparing the area and intensity of shank2-positive puncta from WT neurons permeabilised with either Triton X-100 or digitonin. The method of permeabilisation did not change the area or intensity of the shank2-positive puncta. Images represent immunostaining of cultures from 3 mice of each genotype. Scale bar, 10 μm. Error bars represent SEM.