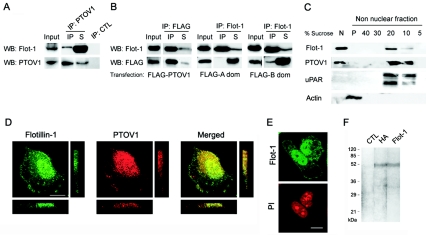
FIG. 1.
PTOV1 interacts with flotillin-1 in mammalian cells. (A) Coimmunoprecipitation of endogenous PTOV1 and flotillin-1 (Flot-1) from PC-3 cells. Total cell lysates were immunoprecipitated with anti-PTOV1 antibody or control rabbit immunoglobulin (IP: CTL). Ten percent of the volume of total cell extracts (Input), the IP, and 10% of the volume of the nonprecipitated fractions (S) were analyzed by Western blotting (WB) with anti-flotillin-1 or anti-PTOV1 antibodies. (B) Flotillin-1 interacts with the second PTOV domain of PTOV1. Total cell lysates from pFLAG-PTOV1, pFLAG A domain (dom), or pFLAG B domain transfections were immunoprecipitated with anti-FLAG or anti-flotillin-1 antibodies. Total cell extracts (10% of the volume [Input]) and the IP and nonimmunoprecipitated fractions (10% of the volume [S]) were analyzed by Western blotting with anti-flotillin-1 or anti-FLAG antibodies. Immunoprecipitation analyses with control rabbit (as shown in panel A) or isotype-matched mouse antibodies were consistently negative and are not shown. (C) PTOV1 and flotillin-1 are distributed in detergent-insoluble floating fractions and also in nuclear fractions. Cells were lysed, and crude nuclear (N) and nonnuclear fractions were extracted. This last fraction was used for sucrose density gradient centrifugation. The fractions (5 to 40% of sucrose) were precipitated by trichloroacetic acid and resuspended in Laemmli buffer, and the entire fractions were analyzed together with the entire crude nuclear extract (N) and the pellet of the gradient (P) from each experiment by Western blotting with antibodies to flotillin-1, PTOV1, uPAR, and actin. (D) Immunocytochemical colocalization of PTOV1 and flotillin-1. Cells were fixed and double labeled for PTOV1 (red) and flotillin-1 (green) endogenous proteins and analyzed by confocal microscopy. Confocal images of the samples were also analyzed along the z axis with sections of 0.6 μm. (E) Nuclei were counterstained with propidium iodide (PI). (F) Immunoprecipitation of flotillin-1 from metabolically labeled PC-3 cells. Cells transfected with pHA-Flot1 were labeled with [35-S]methionine, lysed, and immunoprecipitated with anti-HA, anti-flotillin-1, or a control antibody. Immunoprecipitated proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography. Scale bars, 10 μm.