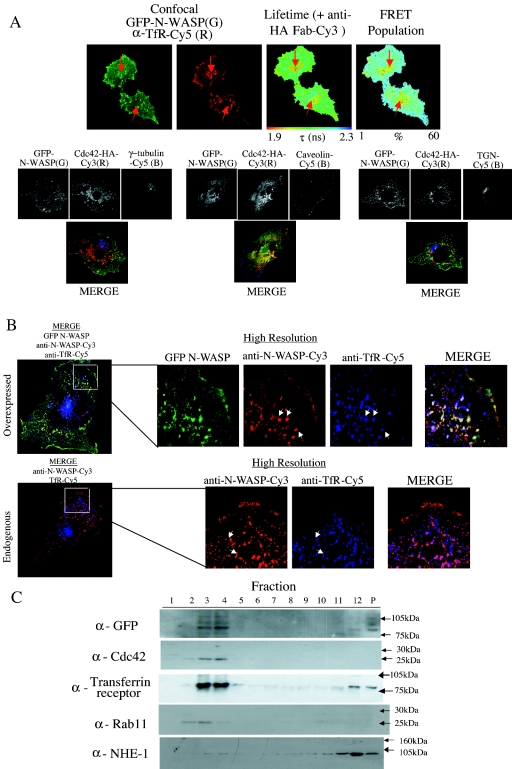
FIG. 6.
Localization of the GFP-N-WASP/Cdc42 complex in cells. (A) MDA-MB-231 cells were microinjected with plasmids encoding GFP-N-WASP and a HA-tagged Cdc42 (plasmid ratio, 1:2), fixed, and dually stained with a Cy3-conjugated anti-HA IgG Fab fragment and an affinity-purified anti-transferrin receptor mouse IgG plus Cy5 secondary conjugate. The GFP intensity image, lifetime map, and distribution of FRET population in cells, as determined by multiphoton FLIM, are shown, along with the corresponding confocal images, which were obtained separately using a Zeiss LSM-510 microscope (G, GFP intensity; R, anti-transferrin receptor Cy5). Where appropriate, the subcellular localization of maximal τ decrease and largest FRET population and its colocalization with a transferrin receptor-positive compartment is indicated by a white arrow. Parallel samples were also dually stained using an anti-γ-tubulin (marker for MTOC) caveolin antibody or anti-TGN (marker for Golgi) antibody, and confocal images alone show that no significant colocalization occurs between GFP-N-WASP and these markers. (B) Confocal imagesof uninjected cells and cells overexpressing GFP-N-WASP on the same coverslip, costained with anti-N-WASP-Cy3 (red) and anti-transferrin receptor-Cy5 (blue). White arrows indicate some areas of colocalization between the N-WASp and transferrin receptor-positive compartments. (C) Biochemical identification of N-WASP-Cdc42 complex localization. Cells were cotransfected with GFP-N-WASP and WT Cdc42-HA, lysed, and subjected to sucrose gradient density fractionation as described in Materials and Methods. All 12 fractions were then run on sodium dodecyl sulfate-polyacrylamide gels, along with 1/10 of the remaining insoluble cell pellet, and Western blotted. The membranes were probed with various antibodies to identify the fractions containing GFP-N-WASP, Cdc42 (both expressed and endogenous Cdc42), transferrin receptor, Rab11, and the plasma membrane-associated Na/H exchanger NHE-1.