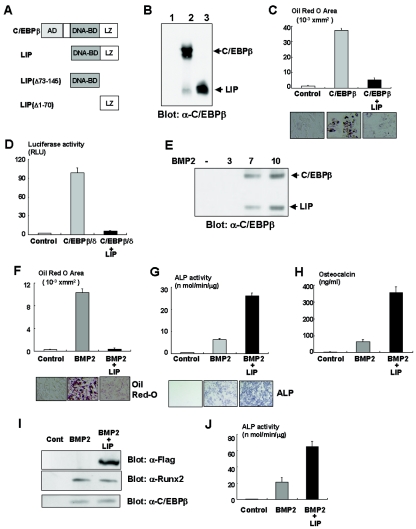
FIG. 4.
LIP inhibits adipocyte differentiation in a dominant-negative fashion. (A) Schematic structure of C/EBPβ, LIP, and its deletion mutants. AD, activation domain; BD, binding domain; LZ, leucine zipper. (B) C3H10T1/2 cells infected with control, C/EBPβ, or LIP adenovirus were analyzed by immunoblotting with anti-C/EBPβ antibody. (C) C3H10T1/2 cells were infected with adenoviruses as indicated in Materials and Methods, incubated for 7 days, and then stained with Oil red O. Oil red O-stained area was measured as described in Materials and Methods. (D) PPARγ gene promoter fused to the luciferase reporter construct and TK-renilla reporter constructs was transfected into C3H10T1/2 cells together with expression vectors as indicated. Luciferase activity of the cell lysates was measured. (E) C3H10T1/2 cells were incubated with BMP2 as indicated, and expression of LIP and C/EBPβ was determined by immunoblotting. (F) C3H10T1/2 cells infected with control or Flag-tagged LIP adenovirus were incubated with BMP2 for 7 days and stained with Oil red O. The Oil red O-stained area was measured. (G and H) C3H10T1/2 cells infected with control or Flag-tagged LIP adenovirus were incubated with BMP2 for 7 days and ALP activity (G) and osteocalcin production (H) were determined. (I) C3H10T1/2 cells infected with control or Flag-tagged LIP adenovirus were incubated with BMP2 for 7 days, and the cell lysates were examined by immunoblotting with anti-Flag, Runx2, or C/EBPβ antibody. (J) C2C12 cells infected with control or Flag-tagged LIP adenovirus were incubated with BMP2 for 7 days, and ALP activity was determined.