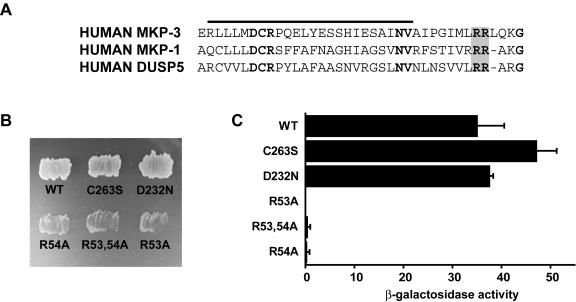
FIG. 3.
A conserved KIM within the amino-terminal noncatalytic domain of DUSP5 mediates specific binding to ERK2. (A) Alignment of sequences within the amino-terminal noncatalytic domains of MKP-3/DUSP6, MKP-1/DUSP1, and DUSP5. Conserved residues within the Cdc25 homology domain (overlined) are shown in bold type, and conserved arginine residues within the KIM are shaded. (B) Yeast two-hybrid assays. Either wild-type (WT) pGBKT7.DUSP5 or the indicated mutants were transformed into PJ69-4A and mated with PJ69-4α expressing pGADT7.ERK2. Yeast diploids expressing both binding domain and activation domain fusions were selected on SD−Leu/−Trp (see the legend to Fig. 1 for an explanation of the medium). Leu- and Trp-positive colonies were restreaked onto SD−Leu/−Trp/−His/−Ade, and protein-protein interactions were assessed by growth on this selective medium. (C) Semiquantitative analysis of the two-hybrid interactions based on the level of induction of the β-galactosidase gene. Assays were performed in triplicate, and means are presented with associated errors.