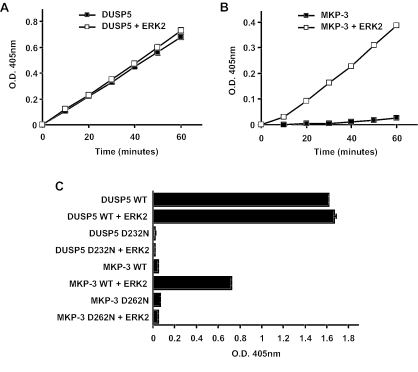
FIG. 5.
DUSP5 does not undergo catalytic activation on incubation with recombinant ERK2, and the conserved general acid residue is fully engaged in catalysis in the absence of substrate. (A) Time-dependent hydrolysis of p-NPP by 2.5 μg of DUSP5 either in the absence (▪) or in the presence (□) of 10 μg of ERK2 was monitored by measuring the change in the optical density (O.D.) at 405 nm. (B) Time-dependent hydrolysis of p-NPP by 2.5 μg of MKP-3 either in the absence (▪) or in the presence (□) of 10 μg of ERK2 was monitored by measuring the change in the optical density at 405 nm. (C) Mutation of the conserved general acid (Asp232) abolishes the basal phosphatase activity of DUSP5, while mutation of the corresponding residue in MKP-3 abolishes only its ERK2-dependent phosphatase activity. Recombinant DUSP5, DUSP5 D232N, MKP-3, or MKP-3 D262N (5 μg) was incubated for 1 h either in the absence or in the presence of 10 μg of ERK2. WT, wild type. Hydrolysis of p-NPP was monitored by measuring the change in the optical density at 405 nm. Assays were performed in triplicate, and means are presented with associated errors.