Abstract
This review discusses strategies for the identification of metabolites in complex biological mixtures, as encountered in metabolomics, which have emerged in the recent past. These include NMR database-assisted approaches for the identification of commonly known metabolites as well as novel combinations of NMR and MS analysis methods for the identification of unknown metabolites. The use of certain chemical additives to the NMR tube can permit identification of metabolites with specific physical chemical properties.
Keywords: : complex mixture analysis, metabolite databases, metabolomics, MS of metabolite mixtures, nanoparticle-assisted metabolomics, NMR of metabolite mixtures, paramagnetic relaxation enhancement
A fundamental characteristic of all living systems is their extraordinarily high complexity at the molecular level [1,2]. This includes both large and small molecules, most of which are part of complex biochemical reaction networks [3–5]. The footprint of all small biological molecules, or metabolites, provides unique information about the state of a living organism, which is a prerequisite for the understanding of the activity of biochemical pathways and their consequences for homeostasis, health and disease, aging, as well as for elucidation of the effect of mutations and other biological, chemical or physical perturbations [6–9]. Over the past few years, the field of metabolomics (also referred to as ‘metabonomics’) has assumed a critical role in the comprehensive characterization of the metabolites of biological systems and their relationship to the biological state of an organism [10–13]. Specifically, metabolomics is providing new insights into the metabolite makeup of biofluids, such as serum and urine, cells, tissues and organs and their role in biochemical pathways [14–16]. Metabolomics allows the identification of biomarkers that are characteristic for particular phenotypes, such as a specific disease, even before the ‘classical’ symptoms occur [17–19]. Metabolomics also promises to be useful for monitoring the treatment of many different health conditions and opens up the prospect for new approaches to wellness and personalized medicine [20,21]. Therefore, the biomedical implications of metabolomics are of paramount significance and are expected to continue to rapidly grow in importance due to the high likelihood within this decade of routine applications for diagnosis and treatment of various conditions and diseases based on a wide range of metabolomics tools [22,23].
MS and NMR spectroscopy are the two major experimental analysis techniques in metabolomics [24,25]. This is primarily because of the exceptional resolution power of both of these techniques to detect individual metabolites in complex mixtures while requiring little or no purification or physical separation of mixture components [26–28]. However, detection of signals alone is often not sufficient for the unambiguous identification of metabolites. In fact, many of the signals observed in MS and NMR spectra of metabolomics studies belong to molecules whose identification is notably difficult [29,30]. Identification of these ‘unknown’ metabolites has been recognized as one of the major challenges in the metabolomics field.
By contrast, for the identification of ‘known’ metabolites, which are those metabolites whose identities are already cataloged in accessible databases, querying of metabolomics databases can be very accurate and efficient [31–33]. Over the recent past, both MS and NMR metabolomics databases have undergone significant expansions of metabolite repositories and enhancements of querying platforms [34–37]. These have led to significantly improved querying results both in terms of an increased true positive rate and lowered false positive rate. A general goal is to expand metabolite data repositories with data of newly discovered metabolites so that database querying can identify an ever-larger number of metabolites in real-world applications.
Other advancements in metabolite identification have occurred by utilizing different instruments for the analysis of the same sample to further increase the accuracy of metabolite identification. Methods combining NMR spectroscopy with MS have received particular attention, because of the high complementarity of these two analytical techniques [38]. These studies integrated NMR and MS by means of multivariate statistical analysis applied to a large number of samples [39–41].
A non-scientific, but non-negligible drawback of combining NMR with MS approaches is the increased cost of high-end NMR and MS instrumentation required, especially when purchased by individual labs. Since the scientific output of metabolomics research now benefits many research areas, universities, government agencies and companies have started to set up core facilities specifically designed for metabolomics research that include both NMR and MS instruments. This not only makes state-of-the-art measurements more affordable and more easily accessible to the larger research community, but also provides opportunities for the development of increasingly standardized protocols for sample preparation and data collection, opening the door for the routine analysis of the same metabolomics sample by complementary analytical techniques [42–45]. These developments have led to a surge in the number of combined NMR/MS metabolomics studies in the literature, a trend expected to continue.
Finally, a significant advance is that new chemical agents have been discovered that selectively interact with certain types of metabolites and thereby either enhance or weaken their NMR and/or MS spectra. Such information not only provides additional physical and chemical information about metabolites such as their electric charge, hydrophobicity and specific functional groups, but it also allows metabolite identification by correlating the signals of the same metabolite across multiple NMR and/or MS spectra. In the following sections, we give an overview of some of the recent advances in metabolite identification and discuss remaining challenges.
Database-assisted metabolite identification
One-dimensional (1D) 1H NMR is the most commonly used approach in NMR-based metabolomics, allowing the analysis of hundreds or even thousands of samples in a relatively short period of time, especially when assisted by automated sample changers [46,47]. However, identification of metabolites in complex mixtures solely based on 1D 1H NMR is a challenge because many peaks tend to overlap in highly crowded spectra as typically encountered in metabolomics [48]. Substantially improved spectral resolution can be obtained by going from 1D to two-dimensional (2D) NMR experiments at the expense of prolonged experimental times [49,50]. In 2D experiments, spin magnetization is transferred between different nuclear spins, which is then depicted in the form of ‘cross-peaks’ when plotting the spectrum against two frequency axes (dimensions). Compared to the 1D spectrum of the same compound, peak overlap in 2D is thereby greatly diminished. Two of the most commonly used 2D NMR experiments in metabolomics are the 2D 13C-1H heteronuclear single quantum coherence spectroscopy (HSQC) experiment [51], which provides correlations between chemical shifts of 1H spins with their directly attached 13C spins, and the 2D 1H-1H total correlation spectroscopy (TOCSY) experiment [52], which provides the chemical shifts of all 1H spins within the same molecule or spin system. The TOCSY experiment contains valuable information about entire groups of resonances that belong to the same molecule, which is not directly available from 1D experiments, but is beneficial both for the identification of known compounds and for the elucidation of the structure of unknown compounds in complex metabolite mixtures. Many metabolomics groups only use 2D NMR in special situations [53,54], whereas others rely almost exclusively on 2D NMR experiments for the accurate and comprehensive identification of metabolites [55–57]. Some of these experiments can benefit from fast NMR methods that speed up the collection of NMR data using a variety of approaches [56,58–59].
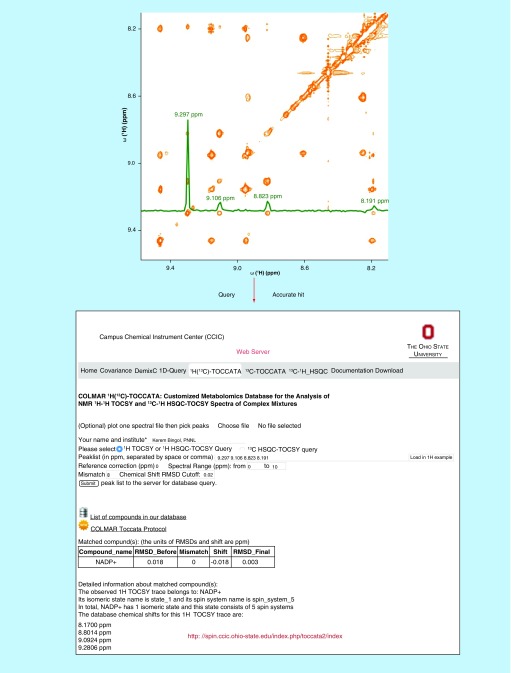
A recent development in NMR-based metabolite identification has been the introduction of customized metabolomics databases that were specifically designed for the querying of 2D TOCSY NMR experiments, which has resulted in significantly increased accuracy of metabolite identification. These include the 13C-TOCCATA customized database [31,60], which specializes on the querying of 13C-13C TOCSY spectra of uniformly 13C labeled metabolomics samples, and the 1H(13C)-TOCCATA customized database [32,61], which permits the querying of 1H-1H TOCSY and 13C-1H HSQC-TOCSY spectra of complex metabolite mixtures at natural 13C abundance. The novel element of these two databases is that they sort the spectral information of each metabolite into its individual spin systems and, if present, its slowly interconverting isomers. Since selected cross-sections of the 2D TOCSY spectrum reflects the 1D spectrum of spin systems (or isomers) rather than the entire 1D spectrum, this increases the accuracy of metabolite identification by more than 35 and 21% over existing 1D 1H and 1D 13C NMR metabolomics databases, respectively [31,32]. The procedure of metabolite identification using the 1H(13C)-TOCCATA customized database is illustrated in Figure 1.
Figure 1. . Metabolite identification by using the customized TOCSY 1H(13C)-TOCCATA database.
In the 2D 1H-1H TOCSY spectrum of Escherichia coli cell lysate (orange), a 1H TOCSY trace displayed as green cross-section is extracted (upper panel). Next, its cross-peaks are queried against the database using the webserver [61]. The query correctly and exclusively assigned the trace to the nicotinamide ring portion of NADP+ (see lower panel depicting a snapshot of the web server).
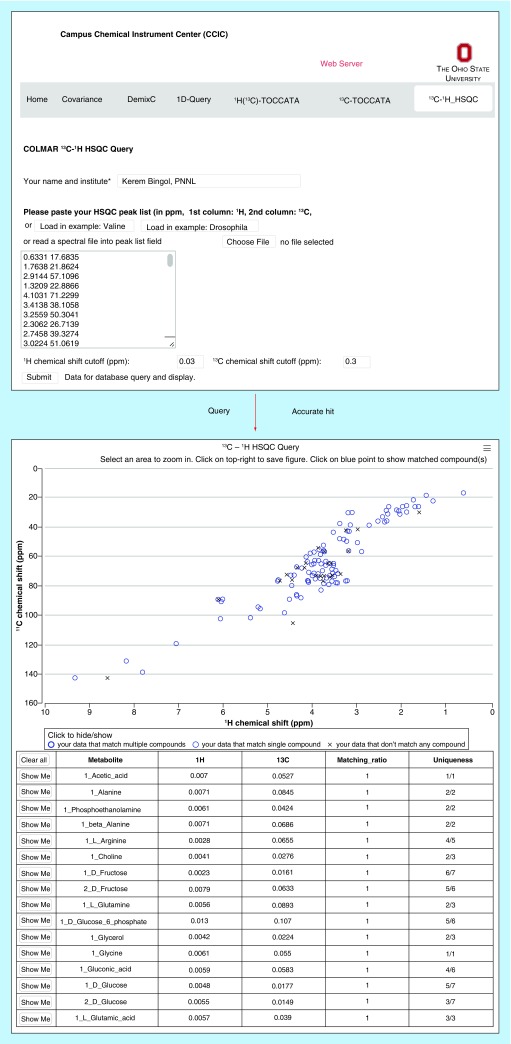
Currently, many researchers use multiple metabolomics NMR databases for the querying of their data in order to maximize the number of identified metabolites in their samples, because the metabolites of different databases overlap only partially. This amounts to an extra effort for the user who will have to go back and forth between databases and their different user interfaces, scoring conventions, etc. In order to facilitate the analysis, a customized NMR metabolomics database for the analysis of 13C-1H HSQC spectra was introduced. This database, termed COLMAR 13C-1H HSQC [33,62], unifies the NMR spectroscopic information of two of the largest public metabolomics databases, namely the Biological Magnetic Resonance Data Bank (BMRB) [63,64] and The Human Metabolome Database (HMDB) [65,66]. COLMAR 13C-1H HSQC sorts HSQC spectra of metabolites into their individual isomeric states, which permits improved querying, because it is isomer-population insensitive, in other words, as long as one of its isomers can be detected, the molecule will be identified. Together with an improved query algorithm, the COLMAR 13C-1H HSQC metabolomics database increases the accuracy of metabolite identification by more than 37% and decreases the false-positive identification rates by more than 82% over existing 13C-1H HSQC metabolomics databases [33]. Example application of COLMAR 13C-1H HSQC to a real-world metabolite sample can be found in Figure 2.
Figure 2. . Screenshots taken from COLMAR 13C-1H HSQC web server. The HSQC peak list with 165 cross-peaks of Drosophila melanogaster metabolite extract (upper panel) is queried against the database.
List of matching compounds returned by the query (lower panel) containing the highest true positive and the lowest false-positive identification rate among 13C-1H HSQC metabolomics web servers. COLMAR 13C-1H HSQC is available for public use at [62].
Reproduced with permission from [33] © American Chemical Society (2015).
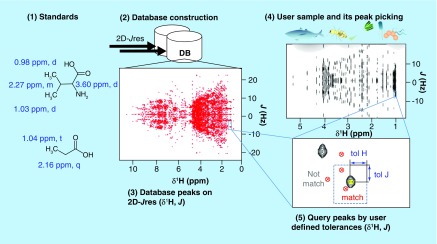
A novel metabolomics database, SpinCouple [67,68] (Figure 3), was introduced for the analysis of 2D J-resolved (Jres) 1H NMR spectra [69]. SpinCouple [67] as well as Birmingham Metabolite Library [70] (BML) [71], allows querying of 2D Jres 1H NMR spectra of complex metabolite mixtures against 2D Jres 1H NMR spectra of metabolite standards. One of the reasons for spectral crowdedness in 1D 1H NMR spectra is the presence of homonuclear proton-proton scalar J-couplings resulting in an increase of the total peak widths. The 2D Jres 1H NMR experiment maps the chemical shift and J-coupling effects onto two orthogonal frequency axes and, hence, leads to an increase in spectral resolution [72]. Since the 2D Jres 1H NMR experiment is a homonuclear 1H experiment, at natural 13C abundance it is more sensitive than a 2D 13C-1H HSQC; however, its resolution is much lower than the one of the 2D 13C-1H HSQC.
Figure 3. . Metabolite identification by using the recent 2D J-resolved NMR database SpinCouple.
The database contains 1H chemical shift and 1H-1H J-coupling information of 598 metabolite standards. It is publically available for querying at [68].
Reproduced with permission from [67] © American Chemical Society (2016).
Querying of experimental 2D NMR spectra against metabolomics databases achieves unambiguous identification of a majority of the cataloged metabolites in model organisms such as Drosophila melonagaster and Escherichia coli [33]. However, this strategy fails when two or more metabolites have very similar chemical shifts, which is often because they have a very similar structure, as is the case, for example, for creatine versus creatine-phosphate and ADP versus ATP metabolites. On the other hand, these metabolites often have different m/z ratios and, hence, it should be possible to differentiate them when including MS information.
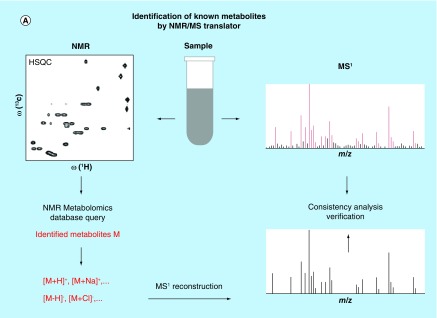
Unfortunately, there has been a lack of strategies for the rapid identification of cataloged metabolites using both NMR and MS spectra as input. Traditionally, cataloged metabolites are identified in the NMR spectrum by using NMR metabolomics databases, whereas cataloged metabolites in the MS spectrum are identified via MS metabolomics databases. Metabolites, along with their names, identified by both methods are then compared with each other [73,74]. This approach is labor intensive and does not fully capitalize on the power of these two analytical approaches even when the two datasets stem from the same sample. In order to address this limitation, a fully automated hybrid NMR/MS approach, the ‘NMR/MS Translator’, [75] has recently been developed. The principle behind this approach is shown in Figure 4A. The NMR/MS Translator first generates metabolite candidates from experimental 1D and/or 2D NMR spectra by NMR database query, which is followed by the automated prediction of the masses (m/z) of all likely ions and adducts of metabolite candidates with their characteristic isotope distributions. The expected m/z ratios are then compared with the experimental MS1 spectrum for the direct assignment of those signals of the mass spectrum that correspond to the metabolites generated from the NMR spectra. In this way, the MS and NMR spectra are simultaneously assigned in a fully automated manner. Furthermore, since chemical shift and accurate mass data were co-analyzed, it substantially increases the accuracy of metabolite identification as compared with entirely separate studies by NMR and MS alone. When the NMR/MS Translator was applied to human urine by combining 2D 13C-1H HSQC with direct infusion ESI-MS spectra, it was able to identify 88 metabolites that have consensus signals in both NMR and MS spectra, whereby molecules that share very similar structures such as creatine versus creatine-phosphate could be easily distinguished. The remarkably large ‘cross-section’ of metabolites identified in this way compares very favorably to completely separate NMR and MS studies, including some of the most extensive studies of this kind reported in the literature [75].
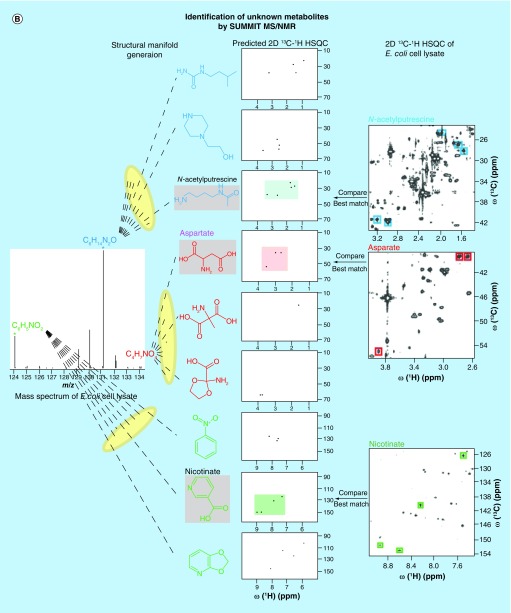
Figure 4. . Recently proposed combined MS/NMR approaches for the rapid and accurate identification of known and unknown metabolites in complex metabolite mixtures.
(A) The NMR/MS Translator strategy allows rapid identification of cataloged metabolites. (B) The SUMMIT MS/NMR strategy allows rapid identification of unknown metabolites.
(A) Reproduced with permission from [75].
(B) Reproduced with permission from [76].
Identification of unknown metabolites
The above-mentioned database-assisted strategies achieve highly accurate and efficient metabolite identification, however, only for those metabolites that have already been compiled in databases. Although excellent progress has been made in the expansion and compilation of NMR metabolomics databases, such as MMCD [77,78], BMRB [63,64], HMDB [65,66] and COLMAR [31–33,79], the further expansion of these databases, while on-going, is time and labor-intensive. The current databases typically contain approximately 300–1000 metabolites, whereas the number of different metabolites in a single organism has been estimated to be in the thousands. Therefore, approaches that rely on databases have clear limitations when it comes to the determination of the entire metabolome of a complex biological system.
In a recent analysis of a pooled human urine sample using the NMR/MS Translator, we found that out of 1012 detected NMR 13C-1H HSQC cross-peaks, only 437 could be assigned to a total of 98 known metabolites [75]. Assuming a similar number of HSQC cross-peaks per compound, another approximate 130 metabolites are estimated to be present; the identities of these molecules, however, cannot be determined due to the lack of database information. Although some of these unknown compounds might have been encountered and possibly even characterized in previous studies, including those in other fields of chemistry, in the context of metabolomics they all classify as unknown compounds as long as their structure and NMR spectroscopic information are not readily available. Discovery of these unknown compounds and their characterization in terms of their chemical composition and structure is a key objective of metabolomics as such compounds participate in biochemical pathways and exert biological roles in health and disease. The traditional approach to the characterization of unknown compounds is based on purification and isolation of each compound followed by spectroscopic and crystallographic characterization [80]. The labor and time-intensive nature of this approach makes it unsuitable for routine investigations of samples with variable complexity encountered in metabolomics, which underlines the need for high-throughput approaches.
2D NMR spectroscopy has been used for the characterization of the backbone topologies of unknown molecules in metabolomics samples toward the elucidation of metabolite structures in complex mixtures. In this way, it was possible to identify 112 individual carbon backbone topologies from a single E. coli cell lysate [81]. In a parallel development, MS of metabolite mixtures has made important inroads in recent years, providing information that is highly complementary to the one derived from NMR. With increasing resolution of mass spectrometers, such as Q-TOF, orbitrap, and FT-ICR, the determination of ‘accurate masses’ of individual metabolites is becoming increasingly routine. This information allows one to deduce the molecular formula of metabolites that underlies each peak in the mass spectrum. However, it is a long way from the knowledge of molecular formulas to the identification of individual molecules, because of the large degeneracy of the structural space (manifold) belonging to a given accurate mass. For example, according to the ChemSpider database [82,83], there are 999 different molecules that have the same mass as tyrosine. With increasing mass, this degeneracy increases exponentially. The mass spectrometric solution to this problem is the measurement of MS/MS spectra of molecular fragments, provided that they are unique for each molecule. However, since fragmentation cannot be accurately predicted, this requires the compilation of MS/MS databases, such as METLIN [84,85], using experimental fragmentation data of individual metabolites. This makes the identification of unknown (i.e., uncataloged) metabolites very challenging by MS alone. By contrast, NMR spectroscopic data can be predicted with reasonable accuracy for a given metabolite structure [86]. NMR chemical shifts are particularly suitable, since they are very sensitive to the nature of the local chemical bonding and hence the chemical shifts will significantly change between most isobaric isomers. Because most metabolites have multiple nuclear spins and, hence, multiple chemical shifts, the overall agreement between experimentally determined chemical shifts and predicted chemical shifts provides an effective filter to identify those metabolites that best fit the experimental data.
Novel combinations of MS and NMR open up new opportunities to address the structure elucidation challenge of unknown metabolites with a transformative potential for the metabolomics field. Very recently, a purification-free hybrid MS/NMR metabolite identification strategy, SUMMIT MS/NMR, has been proposed [76]. The approach first extracts accurate masses of all detected metabolites from high-resolution mass spectra and generates all structures consistent with the derived chemical formulas (‘structural manifold’). The comparison of the predicted NMR spectra of all candidate structures with the experimental NMR spectra of the same sample permits accurate identification of the structures present in the complex mixture of interest. The procedure is sketched for the three metabolites N-acetylputrescine, aspartate and nicotinate in Figure 4B. SUMMIT MS/NMR was applied to E. coli cell extract, where it correctly identified a wide range of different types of metabolites [76]. The results suggest that SUMMIT MS/NMR should become suitable for high-throughput applications for the discovery of new metabolites in biological and biomedical mixtures, without the need for experimental MS and NMR metabolite databases or extensive metabolite purification for the elucidation of the structures of unknown metabolites.
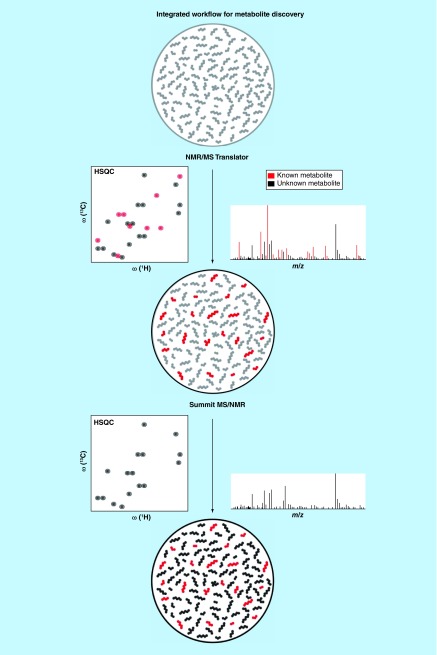
In principle, SUMMIT MS/NMR is capable of elucidating structures of all types of metabolites regardless of being known or unknown, but the approach is most beneficial for the identification of unknown metabolites. In order to systematically apply this protocol to complex biological mixtures, we developed a strategy integrating SUMMIT MS/NMR [76] with the NMR/MS Translator [75] which is depicted in Figure 5. In a first step, the NMR/MS Translator protocol is applied to rapidly identify cataloged metabolites observed both in NMR and MS spectra acquired of the same sample. In this way, a maximal number of NMR and MS signals are assigned to known metabolites, while at the same time, the remaining signals are identified as fingerprints of unknown compounds. Next, for those signals belonging to unknowns, we apply the SUMMIT MS/NMR approach. This two-step strategy reduces the number of experimental m/z ratios that has to be taken into account for chemical shift prediction in the SUMMIT MS/NMR protocol, and therefore significantly reduces the complexity and the computational time for the discovery of unknown metabolites. Application of this integrated strategy to a variety of different metabolomics samples is currently underway in our laboratory.
Figure 5. . The protocol integrating the SUMMIT MS/NMR with the NMR/MS Translator for the systematic and efficient identification of both known and unknown metabolites in complex metabolite mixtures.
Although these approaches can be applied for the comprehensive identification of most, if not all, components of a complex mixture, for certain applications it may be of advantage to focus on a subset of compounds that have the largest potential as biomarkers as their concentrations correlate strongest with the phenotype. Such an approach has the potential for significant speed up as only a subset of all NMR and MS signals need to be analyzed [39–41,44].
Use of chemical agents for improved metabolite identification
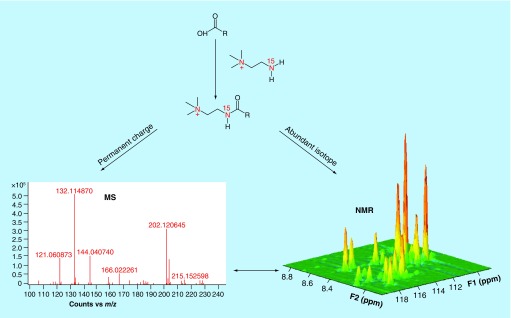
Certain chemical agents are capable of specifically interacting with certain types of metabolites and change their signal intensities both in NMR and MS spectra. This characteristic has recently been utilized to improve metabolite identification. Two 15N labeled agents, cholamine tag [87] and aminooxy probe [88] have been introduced that selectively and covalently attach to carboxyl and carbonyl group containing metabolites, respectively. This makes signals of these metabolites directly visible in 2D 15N-1H HSQC NMR spectra and mass spectra because of the introduction of a permanent charge, thereby linking NMR and MS signals of the modified metabolites. A schematic representation of this strategy is shown in Figure 6.
Figure 6. . A chemo-selective approach to detect the same metabolites using NMR and MS by chemical modification.
15N-labeled cholamine attaches selectively and covalently to carboxyl group containing metabolites and enables their enhanced detection by both MS and NMR.
Reprinted with permission from [87] © American Chemical Society (2013).
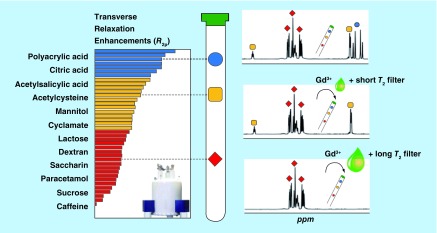
Gadolinium (Gd3+) has been introduced because of its paramagnetic property as a tool for NMR signal suppression of individual components in complex mixtures according to their Gd3+-complexing ability [89]. The metabolites that interact with Gd3+ relax faster (shorter transverse relaxation time T2), which results in the broadening and partial suppression of their signals and might help their identification. However, these effects are mainly restricted to anionic species; they are easier to filter than neutral and cationic species, because of their stronger complexing ability with Gd3+. Recently, an alternative, Carr-Purcell-Meiboom-Gill (CPMG) edited version of the approach has been introduced [90]. CPMG is popular in NMR-based metabolomics for the analysis of blood/serum samples and tissues. With appropriate T2 relaxation delays, CMPG can selectively suppress the signals of macromolecules without significantly affecting metabolite signals [91]. The authors applied the CMPG method to analyze metabolites in the presence of Gd3+. Using appropriate Gd3+ concentrations and T2-relaxation filters, they were able to weaken the NMR signals of different molecular species to a variable degree, thereby assisting the identification of those molecules in complex mixtures (Figure 7) [90].
Figure 7. . The combined use of the paramagnetic spin relaxation agent gadolinium (Gd3+) and CPMG 1H NMR to selectively suppress signals of metabolites in a complex mixture.
A low concentration of Gd3+ combined with a short T2 filter only suppresses the signals from citric acid (blue). Next, a higher Gd3+ concentration along with a longer T2 filter suppresses the signals from acetylcysteine (yellow). The remaining signals in the CPMG 1D 1H NMR spectrum belong to mannitol (red), which is least affected by Gd3+ and the T2 filter.
Adapted with permission from [90] © American Chemical Society (2015).
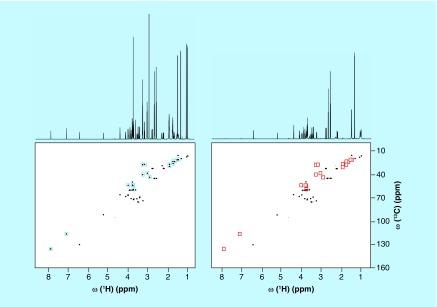
Electrically charged silica nanoparticles have been introduced as a way to differentiate between mixture components in NMR spectra based on their electric charge [92]. When it comes to the detection of electric charge of molecules, NMR spectroscopy is an insensitive technique at constant pH. The new technique addresses this limitation. By adding electrically charged silica nanoparticles of approximately 20 nm diameter to the solution of NMR sample, metabolites of opposite charge bind to the nanoparticles and their NMR signals are substantially weakened or entirely suppressed due to peak broadening caused by the slow rotational tumbling of the nanometer-sized particles [92]. Comparison of the edited spectrum with the original spectrum significantly facilitates analysis and reduces ambiguities in the identification of metabolites. This editing approach has been demonstrated for both anionic and cationic silica nanoparticles, which were able to successfully suppress the signals of positively charged and negatively charged metabolites, respectively, in the NMR spectra of a complex model mixture (Figure 8) as well as of human urine [92].
Figure 8. . Effect of anionic silica nanoparticles on 1D 1H and 2D 13C-1H HSQC spectra of 10-compound metabolite model mixture consisting of lysine, arginine, histidine, citric acid, lactic acid, shikimic acid, alanine, dimethylglycine, glucose and valine 2 mM each (A) without and (B) with anionic silica nanoparticles.
Blue squares highlight the cross-peaks of lysine, arginine, histidine and dimethylglycine that are suppressed in the presence of silica nanoparticles (red squares).
Reproduced with permission from [92] © American Chemical Society (2015).
Conclusion
The future trajectory of metabolomics and its impact on translational biomedicine largely depend on the analytical capabilities of the two main experimental techniques, NMR spectroscopy and MS, as well as optimizing the combination of these techniques to permit rapid identification and quantitation of a large number of metabolites in complex mixtures. Despite recent advances in a variety of areas, a large number of metabolites in common biological samples are still unknown as they are not part of existing metabolomics databases. These unknown metabolites need to be identified and structurally characterized in an accurate and efficient manner. Achievement of these goals requires the development of new methods involving both NMR and MS and their suitable combination along with innovative sample preparation approaches. In order to make these advances accessible to a broadest range of scientists in the biomedical and other fields, easy access to state-of-the-art NMR and MS instruments, the development of integrated, robust and easy-to-use software, web server and databases and the development of optimized sample preparation protocols will be crucial.
Future perspective
If the developments over the recent past is a guide, we anticipate for the near future significant further progress toward the reliable and rapid identification of metabolites in complex mixtures. This includes the emergence of powerful hybrid experimental and database methods that combine multiple techniques into a single platform as well as the development of reagents interact with specific classes of metabolites for their easy monitoring. This will open the door for fully automated analysis with applications to a wide range of areas from medial diagnostics, quality control, synthesis, to food sciences.
Executive summary.
NMR spectroscopy for mixture elucidation
NMR is a very powerful tool for the identification of known and unknown (or unnamed) metabolites in complex mixtures as encountered in metabolomics.
Database-assisted metabolite identification of known compounds
Known compounds can be reliably identified using 2D NMR methods, such as 13C-1H HSQC, for which powerful web servers with associated databases are available for semi-automated analysis.
Identification of unknown metabolites by hybrid approaches
For the identification of unknown compounds, new combinations of NMR with MS have been developed recently that make synergistic use of the mutual strengths of the two techniques.
Use of chemical agents for improved metabolite identification
The use of certain chemical additives to the NMR tube, such as reactive agents, paramagnetic ions, or charged silica nanoparticles, permit the identification of metabolites with specific physical chemical properties.
Acknowledgements
We thank Nancy M Washton and Nancy G Isern for their careful reading of the manuscript.
Footnotes
Financial & competing interests disclosure
This work was supported by the NIH (grant R01 GM 066041 and SECIM grant U24 DK097209-01A1). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Nicholson JK, Wilson ID. Understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson JK, Holmes E, Lindon JC, Wilson ID. The challenges of modeling mammalian biocomplexity. Nat. Biotechnol. 2004;22:1268–1274. doi: 10.1038/nbt1015. [DOI] [PubMed] [Google Scholar]
- 3.Fiehn O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genomics. 2001;2:155–168. doi: 10.1002/cfg.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 5.Fan TW, Lane AN. NMR-based stable isotope resolved metabolomics in systems biochemistry. J. Biomol. NMR. 2011;49:267–280. doi: 10.1007/s10858-011-9484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raamsdonk LM, Teusink B, Broadhurst D, et al. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat. Biotechnol. 2001;19:45–50. doi: 10.1038/83496. [DOI] [PubMed] [Google Scholar]
- 7.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Powers R. The current state of drug discovery and a potential role for NMR metabolomics. J. Med. Chem. 2014;57:5860–5870. doi: 10.1021/jm401803b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scalbert A, Brennan L, Manach C, et al. The food metabolome: a window over dietary exposure. Am. J. Clin. Nutr. 2014;99:1286–1308. doi: 10.3945/ajcn.113.076133. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 11.Dunn WB, Broadhurst DI, Atherton HJ, Goodacre R, Griffin JL. Systems level studies of mammalian metabolomes: the roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011;40:387–426. doi: 10.1039/b906712b. [DOI] [PubMed] [Google Scholar]
- 12.Palmnas MS, Vogel HJ. The future of NMR metabolomics in cancer therapy: towards personalizing treatment and developing targeted drugs? Metabolites. 2013;3:373–396. doi: 10.3390/metabo3020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emwas AH, Luchinat C, Turano P, et al. Standardizing the experimental conditions for using urine in NMR-based metabolomic studies with a particular focus on diagnostic studies: a review. Metabolomics. 2015;11:872–894. doi: 10.1007/s11306-014-0746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckonert O, Keun HC, Ebbels TM, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Choi YH, Verpoorte R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010;5:536–549. doi: 10.1038/nprot.2009.237. [DOI] [PubMed] [Google Scholar]
- 16.Dona AC, Jimenez B, Schafer H, et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014;86:9887–9894. doi: 10.1021/ac5025039. [DOI] [PubMed] [Google Scholar]
- 17.Brindle JT, Antti H, Holmes E, et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat. Med. 2002;8:1439–1444. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- 18.Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 2008;8:617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duarte IF, Diaz SO, Gil AM. NMR metabolomics of human blood and urine in disease research. J. Pharm. Biomed. Anal. 2014;93:17–26. doi: 10.1016/j.jpba.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Clayton TA, Lindon JC, Cloarec O, et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–1077. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- 21.Ramautar R, Berger R, Van Der Greef J, Hankemeier T. Human metabolomics: strategies to understand biology. Curr. Opin. Chem. Biol. 2013;17:841–846. doi: 10.1016/j.cbpa.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson JK, Holmes E, Kinross JM, Darzi AW, Takats Z, Lindon JC. Metabolic phenotyping in clinical and surgical environments. Nature. 2012;491:384–392. doi: 10.1038/nature11708. [DOI] [PubMed] [Google Scholar]
- 23.Lindon JC, Nicholson JK. The emergent role of metabolic phenotyping in dynamic patient stratification. Expert Opin. Drug Metab. Toxicol. 2014;10:915–919. doi: 10.1517/17425255.2014.922954. [DOI] [PubMed] [Google Scholar]
- 24.Bingol K, Brüschweiler R. Two elephants in the room: new hybrid nuclear magnetic resonance and mass spectrometry approaches for metabolomics. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:471–477. doi: 10.1097/MCO.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gowda GA, Raftery D. Can NMR solve some significant challenges in metabolomics. J. Magn. Reson. 2015;260:144–160. doi: 10.1016/j.jmr.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bingol K, Salinas RK, Brüschweiler R. Higher-rank correlation NMR spectra with spectral moment filtering. J. Phys. Chem. Lett. 2010;1:1086–1089. doi: 10.1021/jz100264g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bingol K, Brüschweiler R. Deconvolution of chemical mixtures with high complexity by NMR consensus trace clustering. Anal. Chem. 2011;83:7412–7417. doi: 10.1021/ac201464y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubert J, Nuzillard JM, Purson S, et al. Identification of natural metabolites in mixture: a pattern recognition strategy based on 13C NMR. Anal. Chem. 2014;86:2955–2962. doi: 10.1021/ac403223f. [DOI] [PubMed] [Google Scholar]
- 29.Wishart DS. Advances in metabolite identification. Bioanalysis. 2011;3:1769–1782. doi: 10.4155/bio.11.155. [DOI] [PubMed] [Google Scholar]
- 30.Halabalaki M, Vougogiannopoulou K, Mikros E, Skaltsounis AL. Recent advances and new strategies in the NMR-based identification of natural products. Curr. Opin. Biotechnol. 2014;25:1–7. doi: 10.1016/j.copbio.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Bingol K, Zhang F, Bruschweiler-Li L, Brüschweiler R. TOCCATA: a customized carbon total correlation spectroscopy NMR metabolomics database. Anal. Chem. 2012;84:9395–9401. doi: 10.1021/ac302197e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingol K, Bruschweiler-Li L, Li DW, Brüschweiler R. Customized metabolomics database for the analysis of NMR 1H-1H TOCSY and 13C-1H HSQC-TOCSY spectra of complex mixtures. Anal. Chem. 2014;86:5494–5501. doi: 10.1021/ac500979g. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents the first customized 2D 1H-1H TOCSY and 2D 13C-1H HSQC-TOCSY metabolomics database and query, which significantly improved the accuracy of metabolite identification. The web server is open to the public at [61].
- 33.Bingol K, Li DW, Bruschweiler-Li L, et al. Unified and isomer-specific NMR metabolomics database for the accurate analysis of 13C-1H HSQC spectra. ACS Chem. Biol. 2015;10:452–459. doi: 10.1021/cb5006382. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents the first unified and customized 2D 13C-1H HSQC metabolomics database and query, which significantly improved the accuracy of metabolite identification. The web server is open to the public at [62].
- 34.Ellinger JJ, Chylla RA, Ulrich EL, Markley JL. Databases and software for NMR-based metabolomics. Curr. Metabol. 2013;1:28–40. doi: 10.2174/2213235X11301010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso A, Marsal S, Julia A. Analytical methods in untargeted metabolomics: state of the art in 2015. Front. Bioeng. Biotechnol. 2015;3:23. doi: 10.3389/fbioe.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu F, Mcalpine JB, Lankin DC, et al. 2D NMR barcoding and differential analysis of complex mixtures for chemical identification: the Actaea triterpenes. Anal. Chem. 2014;86:3964–3972. doi: 10.1021/ac500188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clendinen CS, Pasquel C, Ajredini R, Edison AS. 13C NMR metabolomics: INADEQUATE network analysis. Anal. Chem. 2015;87:5698–5706. doi: 10.1021/acs.analchem.5b00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan Z, Raftery D. Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Anal. Bioanal. Chem. 2007;387:525–527. doi: 10.1007/s00216-006-0687-8. [DOI] [PubMed] [Google Scholar]
- 39.Crockford DJ, Holmes E, Lindon JC, et al. Statistical heterospectroscopy, an approach to the integrated analysis of NMR and UPLC-MS data sets: application in metabonomic toxicology studies. Anal. Chem. 2006;78:363–371. doi: 10.1021/ac051444m. [DOI] [PubMed] [Google Scholar]
- 40.Pan Z, Gu H, Talaty N, et al. Principal component analysis of urine metabolites detected by NMR and DESI-MS in patients with inborn errors of metabolism. Anal. Bioanal. Chem. 2007;387:539–549. doi: 10.1007/s00216-006-0546-7. [DOI] [PubMed] [Google Scholar]
- 41.Crockford DJ, Maher AD, Ahmadi KR, et al. 1H NMR and UPLC-MS(E) statistical heterospectroscopy: characterization of drug metabolites (xenometabolome) in epidemiological studies. Anal. Chem. 2008;80:6835–6844. doi: 10.1021/ac801075m. [DOI] [PubMed] [Google Scholar]
- 42.Fan TW, Lorkiewicz PK, Sellers K, Moseley HN, Higashi RM, Lane AN. Stable isotope-resolved metabolomics and applications for drug development. Pharmacol. Ther. 2012;133:366–391. doi: 10.1016/j.pharmthera.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clendinen CS, Stupp GS, Ajredini R, Lee-Mcmullen B, Beecher C, Edison AS. An overview of methods using 13C for improved compound identification in metabolomics and natural products. Front. Plant Sci. 2015;6:611. doi: 10.3389/fpls.2015.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall DD, Lei S, Worley B, et al. Combining DI-ESI-MS and NMR datasets for metabolic profiling. Metabolomics. 2015;11:391–402. doi: 10.1007/s11306-014-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dame ZT, Aziat F, Mandal R, et al. The human saliva metabolome. Metabolomics. 2015;11:1864–1883. [Google Scholar]
- 46.Holmes E, Loo RL, Stamler J, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suhre K, Wallaschofski H, Raffler J, et al. A genome-wide association study of metabolic traits in human urine. Nat. Genet. 2011;43:565–569. doi: 10.1038/ng.837. [DOI] [PubMed] [Google Scholar]
- 48.Clendinen CS, Lee-Mcmullen B, Williams CM, et al. 13C NMR metabolomics: applications at natural abundance. Anal. Chem. 2014;86:9242–9250. doi: 10.1021/ac502346h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bingol K, Brüschweiler R. Multidimensional approaches to NMR-based metabolomics. Anal. Chem. 2014;86:47–57. doi: 10.1021/ac403520j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bingol K, Zhang F, Bruschweiler-Li L, Brüschweiler R. Quantitative analysis of metabolic mixtures by two-dimensional 13C constant-time TOCSY NMR spectroscopy. Anal. Chem. 2013;85:6414–6420. doi: 10.1021/ac400913m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodenhausen G, Ruben DJ. Natural abundance N-15 NMR by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 1980;69:185–189. [Google Scholar]
- 52.Braunschweiler L, Ernst RR. Coherence transfer by isotropic mixing - application to proton correlation spectroscopy. J. Magn. Reson. 1983;53:521–528. [Google Scholar]
- 53.Saric J, Wang Y, Li J, et al. Species variation in the fecal metabolome gives insight into differential gastrointestinal function. J. Proteome Res. 2008;7:352–360. doi: 10.1021/pr070340k. [DOI] [PubMed] [Google Scholar]
- 54.Misawa T, Date Y, Kikuchi J. Human metabolic, mineral, and microbiota fluctuations across daily nutritional intake visualized by a data-driven approach. J. Proteome Res. 2015;14:1526–1534. doi: 10.1021/pr501194k. [DOI] [PubMed] [Google Scholar]
- 55.Gronwald W, Klein MS, Zeltner R, et al. Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine. Kidney Int. 2011;79:1244–1253. doi: 10.1038/ki.2011.30. [DOI] [PubMed] [Google Scholar]
- 56.Guennec AL, Giraudeau P, Caldarelli S. Evaluation of fast 2D NMR for metabolomics. Anal. Chem. 2014;86:5946–5954. doi: 10.1021/ac500966e. [DOI] [PubMed] [Google Scholar]
- 57.Wen H, An YJ, Xu WJ, Kang KW, Park S. Real-time monitoring of cancer cell metabolism and effects of an anticancer agent using 2D in-cell NMR spectroscopy. Angew. Chem. Int. Ed. 2015;54:5374–5377. doi: 10.1002/anie.201410380. [DOI] [PubMed] [Google Scholar]
- 58.Motta A, Paris D, Melck D. Monitoring real-time metabolism of living cells by fast two-dimensional NMR spectroscopy. Anal. Chem. 2010;82:2405–2411. doi: 10.1021/ac9026934. [DOI] [PubMed] [Google Scholar]
- 59.Martineau E, Giraudeau P, Tea I, Akoka S. Fast and precise quantitative analysis of metabolic mixtures by 2D 1H INADEQUATE NMR. J. Pharm. Biomed. Anal. 2011;54:252–257. doi: 10.1016/j.jpba.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 60.http://spin.ccic.ohio-state.edu/index.php/toccata/index COLMAR 13C-TOCCATA: a Carbon TOCSY NMR Metabolomics Database.
- 61.http://spin.ccic.ohio-state.edu/index.php/toccata2/index COLMAR 1H(13C)-TOCCATA: Customized Metabolomics Database for the Analysis of NMR 1H-1H TOCSY and 13C-1H HSQC-TOCSY Spectra of Complex Mixtures.
- 62.http://spin.ccic.ohio-state.edu/index.php/hsqc/index COLMAR 13C-1H HSQC Query.
- 63.Ulrich EL, Akutsu H, Doreleijers JF, et al. BioMagResBank. Nucleic Acids Res. 2008;36:D402–D408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.www.bmrb.wisc.edu Biological Magnetic Resonance Data Bank.
- 65.Wishart DS, Jewison T, Guo AC, et al. HMDB 3.0-The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.www.hmdb.ca The Human Metabolome Database.
- 67.Kikuchi J, Tsuboi Y, Komatsu K, Gomi M, Chikayama E, Date Y. SpinCouple: development of a web tool for analyzing metabolite mixtures via two-dimensional J-resolved NMR database. Anal. Chem. 2016;88:659–665. doi: 10.1021/acs.analchem.5b02311. [DOI] [PubMed] [Google Scholar]
- 68.http://emar.riken.jp/spincpl SpinCouple.
- 69.Aue WP, Karhan J, Ernst RR. Homonuclear broad-band decoupling and 2-dimensional J-resolved NMR-spectroscopy. J. Chem. Phys. 1976;64:4226–4227. [Google Scholar]
- 70.www.bml-nmr.org Birmingham Metabolite Library.
- 71.Ludwig C, Easton JM, Lodi A, et al. Birmingham Metabolite Library: a publicly accessible database of 1D 1H and 2D 1H J-resolved NMR spectra of authentic metabolite standards (BML-NMR) Metabolomics. 2012;8:8–18. [Google Scholar]
- 72.Fonville JM, Maher AD, Coen M, Holmes E, Lindon JC, Nicholson JK. Evaluation of full-resolution J-resolved 1H NMR projections of biofluids for metabonomics information retrieval and biomarker identification. Anal. Chem. 2010;82:1811–1821. doi: 10.1021/ac902443k. [DOI] [PubMed] [Google Scholar]
- 73.Psychogios N, Hau DD, Peng J, et al. The human serum metabolome. PLoS ONE. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bouatra S, Aziat F, Mandal R, et al. The human urine metabolome. PLoS ONE. 2013;8:e73076. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bingol K, Brüschweiler R. NMR/MS Translator for the enhanced simultaneous analysis of metabolomics mixtures by NMR spectroscopy and mass spectrometry: application to human urine. J. Proteome Res. 2015;14:2642–2648. doi: 10.1021/acs.jproteome.5b00184. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents a combined NMR/MS approach that provides rapid and accurate identification of cataloged, in other words, known metabolites detected in both NMR and MS spectra of the same metabolomic sample.
- 76.Bingol K, Bruschweiler-Li L, Yu C, Somogyi A, Zhang F, Brüschweiler R. Metabolomics beyond spectroscopic databases: a combined MS/NMR strategy for the rapid identification of new metabolites in complex mixtures. Anal. Chem. 2015;87:3864–3870. doi: 10.1021/ac504633z. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Introduces a combined NMR/MS approach that allows structure elucidation of unknown metabolites in complex metabolite mixtures. The approach does not require purifications of unknown compounds from the matrix, therefore it provides a platform with the potential for high-throughput discovery of new metabolites.
- 77.http://mmcd.nmrfam.wisc.edu Madison Metabolomics Consortium Database.
- 78.Cui Q, Lewis IA, Hegeman AD, et al. Metabolite identification via the Madison Metabolomics Consortium Database. Nat. Biotechnol. 2008;26:162–164. doi: 10.1038/nbt0208-162. [DOI] [PubMed] [Google Scholar]
- 79.http://spin.ccic.ohio-state.edu/index.php/colmar COLMAR.
- 80.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 81.Bingol K, Zhang F, Bruschweiler-Li L, Brüschweiler R. Carbon backbone topology of the metabolome of a cell. J. Am. Chem. Soc. 2012;134:9006–9011. doi: 10.1021/ja3033058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.www.chemspider.com ChemSpider.
- 83.Pence HE, Williams A. ChemSpider: an online chemical information resource. J. Chem. Educ. 2010;87:1123–1124. [Google Scholar]
- 84.https://metlin.scripps.edu/index.php Scripps Center for Metabolomics.
- 85.Zhu ZJ, Schultz AW, Wang J, et al. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat. Protocols. 2013;8:451–460. doi: 10.1038/nprot.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Willoughby PH, Jansma MJ, Hoye TR. A guide to small-molecule structure assignment through computation of 1H and 13C NMR chemical shifts. Nat. Protoc. 2014;9:643–660. doi: 10.1038/nprot.2014.042. [DOI] [PubMed] [Google Scholar]
- 87.Tayyari F, Gowda GA, Gu H, Raftery D. 15N-cholamine- a smart isotope tag for combining NMR- and MS-based metabolite profiling. Anal. Chem. 2013;85:8715–8721. doi: 10.1021/ac401712a. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Introduces 15N-cholamine tag to facilitate detection of carboxyl group containing metabolites in NMR and MS spectra of human serum and urine.
- 88.Lane AN, Arumugam S, Lorkiewicz PK, et al. Chemoselective detection and discrimination of carbonyl-containing compounds in metabolite mixtures by 1H-detected 15N nuclear magnetic resonance. Magn. Reson. Chem. 2015;53:337–343. doi: 10.1002/mrc.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Introduces 15N-labeled aminooxy probes to facilitate detection of carbonyl group containing metabolites in NMR and MS spectra of lung adenocarcinoma cell line.
- 89.Fernandez-Megia E, Correa J, Novoa-Carballal R, Riguera R. Paramagnetic NMR relaxation in polymeric matrixes: sensitivity enhancement and selective suppression of embedded species (1H and 13C PSR filter) J. Am. Chem. Soc. 2007;129:15164–15173. doi: 10.1021/ja0737117. [DOI] [PubMed] [Google Scholar]
- 90.Correa J, Pinto LF, Riguera R, Fernandez-Megia E. Predicting PSR filters by transverse relaxation enhancements. Anal. Chem. 2015;87:760–767. doi: 10.1021/ac5037186. [DOI] [PubMed] [Google Scholar]; • Combines paramagnetic relaxation agent, gadolinium, with CPMG NMR spectroscopy to selectively filter components in mixtures based on their complexing ability with gadolinium.
- 91.Gowda GAN, Raftery D. Quantitating metabolites in protein precipitated serum using NMR spectroscopy. Anal. Chem. 2014;86:5433–5440. doi: 10.1021/ac5005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang B, Xie M, Bruschweiler-Li L, Bingol K, Brüschweiler R. Use of charged nanoparticles in NMR-based metabolomics for spectral simplification and improved metabolite identification. Anal. Chem. 2015;87:7211–7217. doi: 10.1021/acs.analchem.5b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Introduces electrically charged silica nanoparticles as chemical agents to differentiate mixture components in NMR spectra based on their electric charge.