Fig. 2.
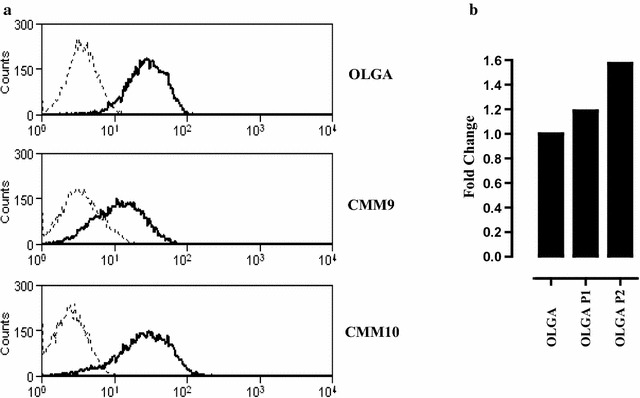
CSPG4 expression in canine melanoma cells and derived-CIC. a CSPG4 expression levels in three canine melanoma cell lines: OLGA [121], CMM9 and CMM10 (kind gift from Dr. Sasaki Nobuo and Dr. Nakagawa Takayuki, Laboratory of Veterinary Surgery, University of Tokyo, Japan). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 50 U/mL penicillin, and 50 μg/mL streptomycin (both from Invitrogen) in humidified incubator at 37 °C under 5% CO2. 2 × 105 cells were incubated with a mix of CSPG4-specific mAb (225.28, VF4-TP108, VF20-TP108 and VF20-VT20; kindly provided by Prof. Soldano Ferrone, Massachusetts General Hospital, Boston, MA, USA) for 1 h at 4 °C. After washing with PBS, cells were incubated with a FITC-conjugated anti-mouse secondary antibodies for 30 min at 4 °C. Flow cytometry was performed with a CyAn ADP (DakoCytomation) and the results were analyzed with Summit 4.2 (DakoCytomation) software. Black lines show CSPG4 expression, while dotted grey lines show the background of cells stained with FITC-conjugated anti-mouse secondary antibody alone. A representative staining of three independent experiments is reported. b For CIC-enrichment, epithelial melanoma cells were detached by using non-enzymatic and mechanical dissociation and plated in ultra-low-attachment flasks at 6 × 104 viable cells/mL in serum-free DMEM-F12 medium supplemented with 20 ng/mL basic fibroblast growth factor (FGF), 20 ng/mL epidermal growth factor (EGF), 5 µg/mL insulin, and 0.4% bovine serum albumin (BSA). Non-adherent spherical clusters of cells (P1), were collected after 7 days and disaggregated using non-enzymatic and mechanical dissociation. P1-derived single-cell suspensions were seeded again at 6 × 104 viable cells/mL to generate non-adherent spherical clusters of cells (P2). 1 μg of RNA extracted from OLGA, P1-OLGA and P2-OLGA was retrotranscribed using RETROscript™ reagents (Ambion) and qPCR was carried out using gene-specific primers (Qiagen). Data were analyzed using SDS software 2.3 (Applied Biosystems). Relative CSPG4 gene expression was quantified using the threshold cycle (CT) value and normalized to housekeeping RNA18S. Relative expression of CSPG4 gene in the P1-OLGA and P2-OLGA compared with OLGA epithelial cells was calculated according to the method of Fold Change (2−(DeltaDelta CT)). Results representative of one out of three independent experiments is reported
