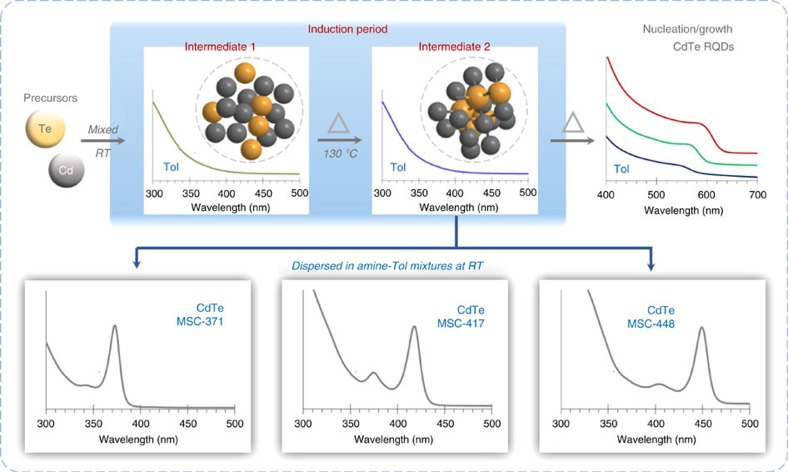
Figure 1. Schematic illustrating the formation pathways of intermediates, magic-sized clusters, and quantum dots.
In traditional synthetic batches leading to colloidal regular quantum dots (RQDs which exhibit bandgap absorption in toluene that redshifts as the size increases), there is a so-called induction period occurring before QD nucleation and growth. With CdTe, during the induction period the development of Intermediate 1 at room temperature (RT) is followed by the formation of Intermediate 2 at elevated temperatures. Both intermediates are ∼1 nm in size, and their properties were monitored by optical absorption spectroscopy, SAXS, MS and NMR. Before monomers are formed, the interaction between the Cd and Te precursors at RT leads to the formation of Intermediate 1. With an increase in the reaction temperature, Cd—Te covalent bonds form, leading to Intermediate 2, after which maintaining a continuous supply of thermal energy causes RQDs to form. These two types of intermediates exhibit featureless absorption in toluene (Tol). However, when Intermediate 2 is dispersed in mixtures of a primary amine and toluene at RT, CdTe MSCs are formed, namely MSC-371, MSC-417, and MSC-448, each as a sole product without the coexistence of NCs of other sizes.