Abstract
Three types of orbitofrontal cortex (OFC) sulcogyral patterns that have been identified in the population, and the distribution of these three types in clinically diagnosed schizophrenic patients has been found to be distinct from the normal population. Schizophrenia is associated with increased levels of social and physical anhedonia. In this study, we asked whether variation in anhedonia in a neurologically normal population is associated with altered sulcogyral pattern frequency. OFC sulcogyral type was classified and anhedonia was measured in 58 normal young adults, and the relationship between OFC sulcogyral type and anhedonia was explored. In line with other studies conducted in chronic schizophrenia, individuals with higher levels of physical anhedonia demonstrated atypical sulcogyral patterns. Individuals with higher physical anhedonia showed a reduced incidence of Type I OFC and an increased incidence of Type II OFC in the left hemisphere compared to individuals with lower physical anhedonia. These findings support the notion that Type I OFC sulcogyral pattern is protective of anhedonia compared to Type II, even in individuals that are not schizophrenic. Overall, these results support the view that symptoms and neural indices typically associated with neuropsychiatric disorders actually reflect quantitative traits that are continuously distributed throughout the general population. Hum Brain Mapp 37:3873–3881, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: physical anhedonia, orbitofrontal cortex, sulcogyral pattern, gray matter volume
INTRODUCTION
One of the defining features of schizophrenia is anhedonia: a diminished capacity, or inability, to experience pleasure—even in typically pleasurable situations. Anhedonia is similarly an essential component to major depressive disorder [Loas et al., 1992]. Although anhedonia is commonly associated with clinical schizophrenia and depression, nonclinical populations also demonstrate variations in hedonic capacity [Bechara et al., 2000; Harvey et al., 2007]. The manifestation of nontransient highly diminished hedonic capacity, or “trait anhedonia” [Blanchard et al., 2001] can be measured using the Chapman Anhedonia Scale [Chapman et al., 1976].
The subjective experience of pleasure (or hedonic experience) originates from brain regions that activate to propel us toward activities that increase our likelihood of surviving and passing on our genetic material—eating, drinking, and having sex. The anticipation and pleasure experience that accompanies the attainment of rewards is driven by a group of neural structures, often referred to as the “reward system,” and includes both subcortical regions (nucleus accumbens, striatum, amygdala) as well as the frontal cortices. The orbitofrontal cortex (OFC) is interesting to the study of individual differences in anhedonia because functional nodes that process reward‐related values in this region also appear to be organized according to the underlying morphology of the OFC. Recent work has shown that individual morphological variability in sulcogyral patterns correlates with the organization of functionally‐defined value signals in the OFC [Li et al., 2015].
While this discovery of an anatomical‐functional relationship between reward experience and OFC morphology indicates that specific value signals are organized according to underlying anatomical constraints, it remains unknown whether differences in sulcogyral pattern type may predict variability in the experience of pleasure more generally.
Research suggests that sulcogyral patterns in the OFC form during neurodevelopment, and remain fixed throughout an individual's life [Armstrong et al., 1995]. Thus OFC sulcogyral patterns tend to remain stable in a given population over time. A 2014 study found that OFC pattern type distribution was atypical in Australian adolescents who were born extremely premature and/or with extremely low birth weight [Ganella et al., 2015], indicating that there may be a period of susceptibility during late fetal gestation where sulcogyral pattern are formed and potentially influenced by nongenetic sources of variability. Another study found that the abnormal expression of OFC sulcogyral types is present in individuals who develop schizophrenia prior to any development of symptoms [Nishikawa et al., 2016]. These findings suggest that morphological abnormalities of the OFC do not arise as a result of the illness, or the antipsychotic medications used to treat it, but rather become fixed at key early stages of development.
These findings are particularly compelling in light of the OFC's involvement in aspects of executive functioning and social‐emotional behavior, and its implications in mental illnesses such as schizophrenia and depression [Bechara et al., 2000; Dolcos et al., 2016; Webb et al., 2016]. Indeed, variability in psychosis has been shown to correlate with the expression in sulcogyral patterns in the OFC. A technique for identifying these patterns was first described by Chiavaras and Petrides [2000], who created a classification system for the three main types of sulcogyral patterns observed in the human OFC based on the continuity of the lateral orbital sulci (LOS) and medial orbital sulci (MOS). In nonclinical populations, Type I patterns tend to be the most common (56% of hemispheres), Type II slightly less common (30% of hemispheres), and Type III relatively rare (around 14% of hemispheres). In 2007, Nakamura et al. observed that the frequency of expression of these patterns differs in populations with schizophrenia, where Type I patterns become significantly less common and rare, Type III patterns increase in frequency [Nakamura et al., 2007]. Such findings were corroborated by other research groups [Nishikawa et al., 2016; Takayanagi et al., 2010]. These studies also found that the presence of Type III sulcogyral patterns in individuals with schizophrenia was associated with poorer cognitive function, lower socioeconomic status, and more severe psychotic symptoms. In contrast, Type I patterns tended to correlate with higher intelligence, better working memory, and decreased expression of psychotic symptoms. Nakamura and colleagues also found evidence that the presence of Type III patterns correlates with increased disorganized symptom ratings in patients with schizophrenia. Until 2013, Type II patterns were not shown to significantly correlate with psychotic symptoms, but Bartholomeusz and colleagues have found that the presence of a Type II pattern in the right hemisphere leads to a highly increased risk of psychosis, especially as compared to Type I pattern expression[Bartholomeusz et al., 2013; Cropley et al., 2015; Whittle et al., 2014]
While OFC sulcogyral patterns have been primarily explored within the context of schizophrenia, OFC gray matter volume has been found to be atypical in other psychiatric disorders that manifest with anhedonic symptoms. For example, Cremers et al. [2011] found that global OFC volume correlated with extraversion, a trait that is protective of depression [Clark et al., 1994; Kotov et al., 2010]. Similarly, higher OFC volume has been found to be protective against depression partially via increased optimism [Dolcos et al., 2016] and OFC cortical volume increases on remission of depression [Phillips et al., 2015]. With this in mind, we anticipated that OFC cortical volume could account for some of the variance in anhedonia in the normal population.
The present study aims to explore a potential link between OFC sulcogyral patterns, gray matter volume, and the manifestation of subclinical anhedonic traits in normal individuals without diagnosed mental illness. We hypothesized that the same sulcogyral patterns that tend to correlate with a diagnosis of schizophrenia would also correlate with the expression of anhedonia, a key symptom of clinical depression and schizophrenia, in a nonclinical population [Pelizza and Ferrari, 2009]. Because most psychological traits exist on a continuum, individuals manifest differing levels of hedonic capacity [Harvey et al., 2007]. The possible protective benefits of the Type I sulcal pattern could be due to either lower mean anhedonia or lower variance and consequently fewer cases of extreme anhedonia. We hypothesized that variation in sulcogyral patterns and cortical volume would predict individual differences in expression of anhedonia, even in individuals who have not been diagnosed with a psychological disorder. Thus, we investigated four possible significant relationships; left and right hemispheric sulcogyral patterns, and both social and physical anhedonia.
METHODS AND MATERIALS
Participants
A group of 58 healthy right‐handed subjects, 30 females and 28 males, were included in data analysis, whose ages ranged from 18‐ to 36‐years old (23.3 ± 4.0 years). Subjects were drawn from an urban population, and their education levels ranged from 13 to 18 years of education (16.0 ± 1.4 years). All participants identified English as their primary language. None of the subjects had a history of neurological or psychiatric disorders as assessed by self‐report. First‐degree relatives were not queried. Informed consent was obtained according to the guidelines of the Institutional Review Board of Temple University, and participants received monetary compensation for participation in the experiment.
Measures
The revised physical anhedonia scale
The Revised Physical Anhedonia Scale (PAS) [Chapman et al., 1976] is a self‐report measure, containing 61 True‐False items, which assesses deficits in the ability to experience pleasure from typically pleasurable stimuli (for example, sex and food). The PAS yields scores between 0 and 61, with higher scores indicating more severe physical anhedonia.
The revised social anhedonia scale
The Revised Social Anhedonia Scale [Chapman et al., 1976] is a self‐report measure, containing 40 True‐False items, which assesses deficits in the ability to experience pleasure from nonphysical stimuli such as social interactions. Scores range from 0 to 40, with higher scores indicating more severe social anhedonia.
Image Acquisition
MRI scanning was conducted at Temple University Hospital on a 3.0 T Siemens Verio scanner (Erlangen, Germany) using a Siemens 12‐channel phased‐array head coil. High‐resolution anatomical images (T1‐weighted 3D MPRAGE) were also collected for each participant with the following parameters: 160 axial slices, 1 mm slice thickness, TR = 1,900 ms, TE = 2.93 ms, inversion time = 900 ms, flip angle = 9°, FOV = 256 mm.
Sulcal Tracing
To analyze the OFC sulcal patterns, the T1 anatomical images were normalized by first stripping non‐brain tissue using FMRIB Software Library Brain Extraction Tool [Smith, 2002], then aligned along the anterior commissure‐posterior commissure plane to adjust for head tilt (using FMRIB Linear Image Registration Tool, FLIRT [Jenkinson and Smith, 2001; Jenkinson et al., 2002] after registration to an Montreal Neurological Institute (MNI) template, and resampled into 1 mm cubic voxels.
The OFC sulcal patterns were identified from the normalized images using the software ITK‐SNAP [Yushkevich et al., 2006] and classified according to the criteria used in previous analysis of OFC sulcogyral patterns and schizotypy [Ganella et al., 2015; Lavoie et al., 2014]; see Table 1. The morphology of the orbitofrontal sulci was categorized into three main types (Type I, II, and III) in each hemisphere based on the continuity of the MOS and LOS, respectively. [Chiavaras and Petrides, 2000].
Table 1.
The determining criteria for OFC sulcal pattern classification
| Type | Rostral and caudal MOS connected? | Rostral and caudal LOS connected? |
|---|---|---|
| I | No | Yes |
| II | Yes | Yes |
| III | No | No |
MOS = medial orbital sulci; LOS = lateral orbital sulci.
A sulcus was determined to be discontinuous if the sulcus was absent for three adjacent coronal slices separating the rostral and caudal portions. Each subject's OFC sulcal pattern was independently evaluated by 3 raters, and the inter‐rater reliability of classification was κ = 0.71.
Voxel‐Based Morphometry
Preprocessing of T1 structural images was conducted using the voxel‐based morphometry (VBM) toolbox in SPM12 (Wellcome Department of Imaging Neuroscience Group, UK; http://www.fil.ion.ucl.ac.uk/spm). Each structural image was DARTEL‐normalized (diffeomorphic anatomical registration through exponentiated lie algebra) to MNI space, and the images were subsequently segmented into gray matter, white matter, and cerebrospinal fluid. Normalized gray matter images were smoothed with a 10 mm full‐width at half‐maximum Gaussian kernel [Ashburner and Friston, 2000]. Using NIFTI masks provided by Thorsten Kahnt (described below), the gray matter volumes of each OFC mask region were computed using the MarsBaR toolkit within SPM12 [Brett et al., 2002]. Values for each individual gray matter region was then entered into higher level statistical analysis using SPSS.
Definition of Regions of Interest
Following a detailed functional parcellation of the OFC [Kahnt et al., 2012], we selected the OFC region of interest (ROI) using labels generated from a connectivity‐based parcellation map of the OFC. This parcellation is generated using an unsupervised clustering technique on a set of resting‐state data. This method resulted in six clusters, corresponding to (1) medial, (2) posterior‐central, (3) central, and (4–6) lateral OFC in each hemisphere (total of 12 regions bilaterally). The resulting ROIs included coverage of voxels in the left and right medial and lateral OFC [Tzourio‐Mazoyer et al., 2002] (Fig. 1A). All other voxels (i.e., most gray matter voxels except for the OFC) as well as the box covering the midbrain (see above) were defined as the rest of the brain. Traditional OFC parcellations only specify a medial–lateral distinction, but parcellations based on cytoarchitecture [Mackey and Petrides, 2010; Ongür et al., 2003] suggest a more detailed parcellation of the OFC than just a medial–lateral distinction [Kahnt et al., 2012]. This parcellation of the human OFC was chosen because was the optimal solution emerging from rigorous analysis that considered the presence of between two and ten distinct clusters of activity in the OFC.
Figure 1.
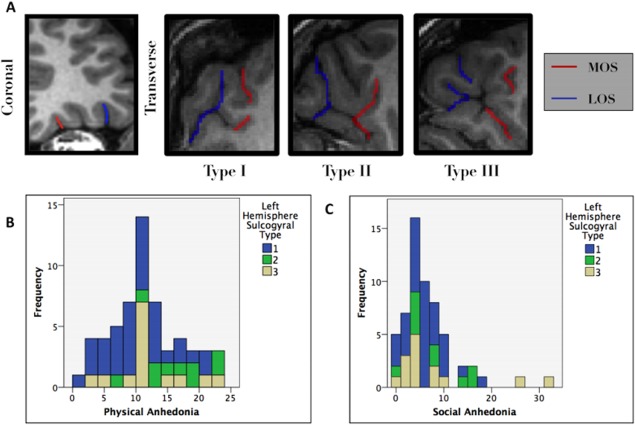
(A) Left hemisphere OFC sulcogyral characterization based on the connectedness of two sulci, the medial orbital sulci (MOS) and the lateral orbital sulci (LOS). (B) Left hemisphere type was significantly correlated with physical anhedonia scores. Type II corresponded with higher physical anhedonia scores compared to Types I and III. Type I sulcogyral pattern was associated with lower mean Physical Anhedonia as compared to Types II and III. (C) Additionally, Type I was associated with lower variability in Social Anhedonia. This suggests a possible protective effect of Type I pattern. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Statistical Analyses
Statistical analyses were performed using SPSS (IBM SPSS 21.0 for PC, SPSS, Chicago, IL). Group differences in the demographic variables age and gender were analyzed with an analysis of covariance (ANCOVA) and Pearson's χ 2 statistics, respectively. The main research hypotheses were assessed using analyses of covariance to look at the group effect of sulcogyral pattern type distribution on anhedonia and within group variance of anhedonia. There were two subjects that were excluded due to outlying social and physical anhedonia levels. Post hoc tests for multiple comparisons were conducted using the False Discovery Rate correction [Benjamini and Hochberg, 1995] with α set at 0.05. This correction involves establishing thresholds of statistical significance such that the expected proportion of false discoveries is lesser than or equal to the family wise error rate. The False Discovery Rate provides a substantial increase in statistical power compared to the Bonferroni correction [Glickman et al., 2014]. We hypothesized any one of four possible significant relationships; either left or right hemisphere and either social or physical anhedonia. Consequently, findings were corrected for four correlations to adjust for multiple comparisons. Additional analyses were conducted on the inter‐hemispheric distribution of pattern types using χ 2 statistics.
RESULTS
Sulcal Tracing Inter‐Rater Reliability
Interclass inter‐rater reliability was κ = 0.71. Left hemisphere interclass classification was slightly more consistent than right hemisphere classification (see Table 2).
Table 2.
The relationship between OFC sulcogyral pattern type and anhedonia was investigated by conducting an ANCOVA
| Type I | Type II | Type III | Statistical test | |
|---|---|---|---|---|
| Left hemisphere | ||||
| N (%) | 34 (57%) | 10 (17%) | 14 (24%) | Reliability, κ = 0.75 |
| Physical Anhedonia, mean +/− (SD) | 9.7 ± 5.1 | 15.5 ± 5.1 | 11.6 ± 5.4 | ANCOVA, P = 0.009 |
| Social Anhedonia, mean +/− (SD) | 5.5 ± 3.8 | 7.3 ± 5.4 | 7.2 ± 9.3 | ANCOVA, P = 0.474 |
| Right hemisphere | ||||
| N (%) | 24 (41%) | 22 (38%) | 12 (21%) | Reliability, κ = 0.67 |
| Physical Anhedonia, mean +/− (SD) | 11.2 ± 5.9 | 11.0 ± 4.6 | 11.4 ± 6.7 | ANCOVA, P = 0.897 |
| Social Anhedonia, mean +/− (SD) | 6.1 ± 6.4 | 5.9 ± 4.4 | 7.2 ± 7.0 | ANCOVA, P = 0.949 |
The ANCOVA controlled for the age, gender, and intracranial volume for the left and right hemispheres of the subjects.
Distribution of patterns in this cohort can be seen in Table 2. The right hemispheres in our sample were more atypical both in pattern type distribution compared to previous findings. There were no significant differences between the average age, gender prevalence, and intracranial volume of the three sulcogyral patterns in both the left and right hemispheres. Although there were no significant differences, we still controlled for age, gender, and intracranial volume in the ANCOVA to focus on the relationship between OFC Sulcogyral type and Anhedonia, and to minimize statistical noise.
Anhedonia and Sulcal Patterns
Contrary to our expectations, there were no significant differences between the three pattern types and mean social anhedonia scores in either hemisphere (F(2, 54) = 0.757, P > 0.05, F(2, 54) = 0.176, P > 0.05, respectively). However, Levene's test indicates that left Hemisphere Type III patterns had significantly higher variability in social anhedonia while Type I was associated with significantly lower variability F(2,55) = 3.611, P < 0.05.
There were significant differences between the three pattern types in the left hemisphere and mean physical anhedonia scores F(2, 54) = 5.117, P < 0.01. The left hemisphere Type I sulcogyral pattern was associated with lower physical anhedonia t(56) = 2.519, P < 0.05, as seen in Figure 1B. Left Hemisphere Type II was associated with higher physical anhedonia than both Type I and Type III.
Correcting for multiple comparisons via the FDR correction, the relationship between left hemisphere Type I sulcogyral pattern and lower physical anhedonia was still significant (P < 0.0125), but the relationship between left hemisphere Type I and lower variance in social anhedonia was not (P > 0.025).
Anhedonia and OFC VBM
While our previous analysis shows a relationship between sulcogyral pattern type and anhedonic traits, we sought to better understand whether differences in gray matter volume actually underlay this relationship. The OFC consists of Brodmann areas 11 and 47 (and sometimes 10 and 13). Recent analysis further divided the OFC by establishing local connectivity profiles based on the resting state connectivity of OFC subregions to the rest of the brain [Kahnt et al., 2012]. Their analysis yielded six nonoverlapping and functionally distinct subregions, which are thought to be related to underlying differences in cytoarchitecture. Consequently our analysis considered the gray matter volume of 12 subregions of the OFC. Of the 12 regions inspected (six per hemisphere), the only statistically significant effect was between the left hemisphere's Region 3 (see Fig. 2B), referred to as the central OFC by Kahnt et al. [2012], and physical anhedonia r(56) = 0.324, P < 0.05, controlling for age, gender, and intracranial volume. Correcting for multiple comparisons via the FDR correction, the relationship between Left Hemisphere Region 3 Gray matter volume and Physical Anhedonia was not significant (P = 0.013; FDR correction threshold: P < 0.002).
Figure 2.
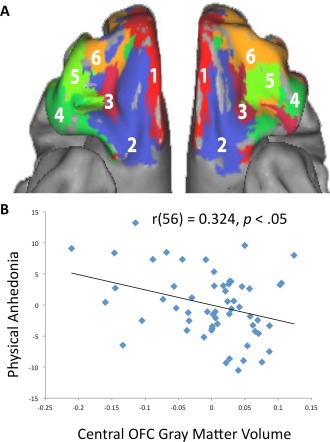
(A) Parcellation map of the OFC based on functional connectivity. Figure reproduced with permission from Kahnt et al. [2012]. (B) OFC gray matter volume in the left central OFC (Region 3 from Figure 2A) was significantly correlated with physical anhedonia scores (controlling for age, gender, and intracranial volume). [Color figure can be viewed at http://wileyonlinelibrary.com.]
OFC Sulcal Patterns and VBM
We next tested the hypothesis that sulcal pattern type was associated with gray matter volume in subregions of the OFC. A possible explanation for the relationship between OFC sulcal patterns and subclinical anhedonia was that it was mediated by regional OFC gray matter volume. If so, then both OFC sulcal pattern and OFC gray matter volume might both simply be indicators of genetically‐derived atypical morphology or early neurodevelopmental health. There was no significant relationship between OFC sulcal pattern and gray matter volume in any of the six regions, F(2, 54) < 3.17, all P's > 0.05), implying that the variation in physical anhedonia accounted for by the left OFC sulcal pattern is not mediated by left central OFC gray matter volume.
DISCUSSION
The results of our study show that individual differences in sulcogyral patterning account for a significant part of the variation in physical anhedonia scores. More specifically, left hemisphere Type II OFC sulcogyral patterns were associated with a decreased ability to experience pleasure (e.g., higher physical anhedonia scores), even when variation caused by age, gender, and intracranial volume were controlled. This is in contrast to the earlier literature that finds Type III patterns to be significantly more associated with psychopathologies [Lavoie et al., 2014; Nakamura et al., 2007; Nishikawa et al., 2016; Takayanagi et al., 2010]. Our finding is in agreement with the relatively recent group of studies where the Type III pattern was more prevalent in the population than expected, and where the OFC Sulcogyral pattern Type II is associated with characteristics of psychopathology14DDI. Higher physical anhedonia scores were also associated with lower OFC cortical volume within the central OFC in the left hemisphere. This is in contrast to a previous finding where monetary rewards caused activation in the central OFC [Li et al., 2015]. As demonstrated in previous literature [Lacerda et al., 2007; McNamara et al., 2007; Meador‐Woodruff et al., 1997; Phillips et al., 2015; Takayanagi et al., 2010; Webb et al., 2016], the OFC likely plays a role in several behaviors relevant to schizotypy and depressive tendencies, such as decision‐making and the valuation of reward. Moreover, deficits in an individual's neurological reward system can manifest as a general lack of interest or pleasure, that is, anhedonia. We hypothesized that the same OFC sulcogyral patterns that are more prevalent among schizophrenics would also correlate with anhedonia. This suggests that sulcogyral patterns could be a predictive factor for anhedonia, and consequently a predictive factor for psychopathy and depression in the normal population with further investigation.
Region 3, the central OFC cluster depicted in Figure 2A, is functionally connected with the anterior insula, ventrolateral PFC, dorsal anterior cingulate, and striatum [Kahnt et al., 2012]. The anterior insular cortex is implicated in several functions, including reward and pain processing [Apkarian et al., 2005; Price, 2000]. The striatum, particularly the ventral striatum, is considered to be a reward center [Gregorios‐Pippas et al., 2009; O'Doherty et al., 2006]. A simple interpretation for the importance of the gray matter volume of the central OFC is that the central OFC acts as a site of integration of information from its previously mentioned sites of co‐activation, even at rest [Andrews‐Hanna et al., 2014; Belcher et al., 2013]. Consequently, the structural integrity of the central OFC may profoundly influence construction of the cognitive map, an associative structure for generating predictions of value from specific events. This map's contours might be in part marked by physical reward that is, physical hedonia [Stalnaker et al., 2015]. If so, then an abnormality in this region could lead to disrupted processing of physical rewards, leading to increased physical anhedonia.
Anhedonia
Patients with schizophrenia have noticeably increased difficulty in experiencing pleasurable feelings from social and physical stimuli. Like many psychological traits, there is significant variation in anhedonia in nonclinical samples as well. Although our findings did not reveal an association between OFC sulcogyral pattern type and social anhedonia, we did find significant differences in OFC sulcogyral pattern type and physical anhedonia.
Kringelbach's [2005] review on the OFC and hedonic experience offers a plausible interpretation of our results [Kringelbach, 2005]. Because the OFC has been found to be involved in the representation of the reward value, expected reward value, and subjective pleasantness of food and other reinforcers [Gottfried et al., 2003; Kringelbach et al., 2003; O'Doherty et al., 2001], the OFC is a critical component of the evaluation of the hedonic quality of an experience. Li et al. [2015] found posterior activation on the MOS of the OFC for sexual stimuli, and this activation was consistently on the posterior portion of the MOS in Type I and Type II morphologies [Li et al., 2015]. Sexual stimuli and physical stimuli are both generally categorized as primary rewards; thus, a structural difference in the posterior MOS where primary rewards might be processed suggests that the difference morphology could underlie a difference in processing primary rewards in general, including general physical pleasure
The region identified in our analyses corresponds to central OFC. A functional connectivity study found that this OFC subregion showed negative connectivity to a region within the midbrain and positive connectivity to the ventral striatum. The authors suggest that this region of the OFC may have a role in reward learning based on the fact that dopaminergic neurons in the midbrain innervate the striatum and OFC and constitute a teaching signal used in reward learning [Kahnt et al., 2012]. We speculate that physical anhedonia constitutes a constitutively depressed state of reward learning potentially caused by early morphological changes in posterior‐central OFC.
Laterality
Previous studies have reported divergent findings regarding the behavioral effect of the presence of OFC sulcogyral pattern types in either the right or left hemisphere. Accordingly, we did not have a priori predictions concerning the laterality of pattern type expression and anhedonia. Our results showed a significant relationship between sulcal pattern type and anhedonia only in the left hemisphere. We interpret the specificity of left‐lateralization in these findings with caution, given that previous work has found either left‐ or right‐lateralized links between atypical sulcogyral patterns and schizophrenic traits [Bartholomeusz et al., 2013; Ganella et al., 2015; Lavoie et al., 2014]. Additionally, the sulcogyral pattern distribution in our sample resembles Li et al. [2015] but is dissimilar to samples from other studies in that we had a relatively high number of type III OFC sulcogyral individuals, particularly for the left hemisphere (17.2% of sample).
Limitations
It is important to note that all previous studies using the technique used in this article recruited a heterogeneous participant pool who varied in their handedness and age. Our sample was more homogeneous and restricted to right handers, potentially accounting for observed differences in laterality.
In addition, it is possible that we did not find an association with social anhedonia because our sample was underpowered. Last, we were also unable to determine which sulcal pattern groups were driving relationship between central OFC gray matter volume and physical anhedonia due to inadequate statistical power. Future studies involving nonclinical populations should recruit a larger population to increase its ability to discern smaller effects. Additionally, the population should include both right and left‐handed participants and use handed‐ness as a covariate to investigate laterality effects more comprehensively.
Conclusions
The relationship between trait levels of anhedonia in nonclinical individuals and the sulcogyral morphology of their OFC is relatively unexplored. The tendency to experience decreased pleasure to physical stimuli with the Type II sulcal pattern in the left hemisphere and decreased left central OFC cortical volume suggests the possibility of developing an additional diagnostic metric based on OFC morphology that could help to identify susceptibility to physical anhedonia and aid in the identification of individuals who are at greater risk of developing psychotic or depressive illnesses.
ACKNOWLEDGMENTS
We would like to thank Dr. Thorsten Kahnt for kindly providing us with the optimized OFC parcellation map from his 2012 paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors declare no conflicts of interest.
REFERENCES
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain Lond Engl 9:463–484. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K (1995): The ontogeny of human gyrification. Cereb Cortex 5:56–63. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry–the methods. NeuroImage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz CF, et al. (2013): Sulcogyral patterns and morphological abnormalities of the orbitofrontal cortex in psychosis. Prog Neuropsychopharmacol Biol Psychiatry 44:168–177. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR (2000): Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10:295–307. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y, Silva AC, Stein EA (2013): Large‐scale brain networks in the awake, truly resting marmoset monkey. J Neurosci 33:16796–16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300. [Google Scholar]
- Blanchard JJ, Horan WP, Brown SA (2001): Diagnostic differences in social anhedonia: A longitudinal study of schizophrenia and major depressive disorder. J Abnorm Psychol 110:363–371. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB (2002): Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage 16:S497. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML (1976): Scales for physical and social anhedonia. J Abnorm Psychol 85:374–382. [DOI] [PubMed] [Google Scholar]
- Chiavaras MM, Petrides M (2000): Orbitofrontal sulci of the human and macaque monkey brain. J Comp Neurol 422:35–54. [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S (1994): Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol 103:103–116. [PubMed] [Google Scholar]
- Cremers H, van Tol M‐J, Roelofs K, Aleman A, Zitman F, van Buchem MA, Veltman DJ, van der Wee NJA (2011): Extraversion is linked to volume of the orbitofrontal cortex and amygdala. PLoS One 6:e28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley VL, Bartholomeusz CF, Wu P, Wood SJ, Proffitt T, Brewer WJ, Desmond PM, Velakoulis D, Pantelis C (2015): Investigation of orbitofrontal sulcogyral pattern in chronic schizophrenia. Psychiatry Res Neuroimaging 234:280–283. [DOI] [PubMed] [Google Scholar]
- Dolcos S, Hu Y, Iordan AD, Moore M, Dolcos F (2016): Optimism and the brain: trait optimism mediates the protective role of the orbitofrontal cortex gray matter volume against anxiety. Soc Cogn Affect Neurosci 11:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella EP, Burnett A, Cheong J, Thompson D, Roberts G, Wood S, Lee K, Duff J, Anderson PJ, Pantelis C, Doyle LW, Bartholomeusz C; Victorian Infant Collaborative Study Group (2015): Abnormalities in orbitofrontal cortex gyrification and mental health outcomes in adolescents born extremely preterm and/or at an extremely low birth weight. Hum Brain Mapp 36:1138–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman ME, Rao SR, Schultz MR (2014): False discovery rate control is a recommended alternative to Bonferroni‐type adjustments in health studies. J Clin Epidemiol 67:850–857. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ (2003): Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301:1104–1107. [DOI] [PubMed] [Google Scholar]
- Gregorios‐Pippas L, Tobler PN, Schultz W (2009): Short‐term temporal discounting of reward value in human ventral striatum. J Neurophysiol 101:1507–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M (2007): Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non‐clinical subjects. Mol Psychiatry 12:703, 767–775. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD (2012): Connectivity‐based parcellation of the human orbitofrontal cortex. J Neurosci Off J Soc Neurosci 32:6240–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D (2010): Linking ‘big’ personality traits to anxiety, depressive, and substance use disorders: A meta‐analysis. Psychol Bull 136:768–821. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005): The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci 6:691–702. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C (2003): Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 13:1064–1071. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Hardan AY, Yorbik O, Vemulapalli M, Prasad KM, Keshavan MS (2007): Morphology of the orbitofrontal cortex in first‐episode schizophrenia: Relationship with negative symptomatology. Prog Neuropsychopharmacol Biol Psychiatry 31:510–516. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Bartholomeuz CF, Nelson B, Lin A, McGorry PD, Velakoulis D, Whittle SL, Yung AR, Pantelis C, Wood SJ (2014): Sulcogyral pattern and sulcal count of the orbitofrontal cortex in individuals at ultra high risk for psychosis. Schizophr Res 154:93–99. [DOI] [PubMed] [Google Scholar]
- Li Y, Sescousse G, Amiez C, Dreher JC (2015): Local morphology predicts functional organization of experienced value signals in the human orbitofrontal cortex. J Neurosci 35:1648–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G, Salinas E, Guelfi JD, Samuel‐Lajeunesse B (1992): Physical anhedonia in major depressive disorder. J Affect Disord 25:139–146. [DOI] [PubMed] [Google Scholar]
- Mackey S, Petrides M (2010): Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. Eur J Neurosci 32:1940–1950. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, Stanford KE (2007): Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: Gender differences and partial normalization with antipsychotic medications. Schizophr Res 91:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador‐Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ (1997): Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex: Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry 54:1089–1095. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, Levitt JJ, Hsu L, Kawashima T, Niznikiewicz M, Shenton ME (2007): Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain J Neurol 130:693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y, Takahashi T, Takayanagi Y, Furuichi A, Kido M, Nakamura M, Sasabayashi D, Noguchi K, Suzuki M (2016): Orbitofrontal sulcogyral pattern and olfactory sulcus depth in the schizophrenia spectrum. Eur Arch Psychiatry Clin Neurosci 266:15–23. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F (2001): Representation of pleasant and aversive taste in the human brain. J Neurophysiol 85:1315–1321. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ (2006): Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron 49:157–166. [DOI] [PubMed] [Google Scholar]
- Ongür D, Ferry AT, Price JL (2003): Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460:425–449. [DOI] [PubMed] [Google Scholar]
- Pelizza L, Ferrari A (2009): Anhedonia in schizophrenia and major depression: State or trait? Ann Gen Psychiatry 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P (2015): A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment‐resistant depression. Int J Neuropsychopharmacol 18(8):pyv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD (2000): Psychological and neural mechanisms of the affective dimension of pain. Science 288:1769–1772. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Human Brain Mapping 17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G (2015): What the orbitofrontal cortex does not do. Nat Neurosci 18:620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, Masuda N, Mozue Y, Nakamura K, Kawasaki Y, Itokawa M, Sato Y, Yamasue H, Kasai K, Okazaki Y, Suzuki M (2010): Volume reduction and altered sulco‐gyral pattern of the orbitofrontal cortex in first‐episode schizophrenia. Schizophr Res 121:55–65. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, Fava M, McGrath PJ, Weissman M, Parsey R, Kurian BT, Adams P, Weyandt S, Trombello JM, Grannemann B, Cooper CM, Deldin P, Tenke C, Trivedi M, Bruder G, Pizzagalli DA (2016): Neural correlates of three promising endophenotypes of depression: Evidence from the EMBARC study. Neuropsychopharmacology 41:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Bartholomeusz C, Yücel M, Dennison M, Vijayakumar N, Allen NB (2014): Orbitofrontal sulcogyral patterns are related to temperamental risk for psychopathology. Soc Cogn Affect Neurosci 9:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006): User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 31:1116–1128. [DOI] [PubMed] [Google Scholar]


