Fig. 2.
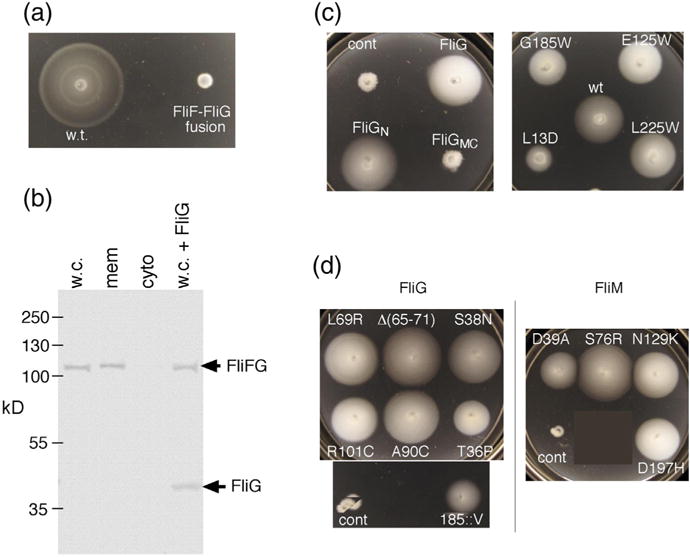
Properties of the original FliF–FliG fusion strain. The strain has a 7-nt deletion near the end of fliF that brings it into frame with fliG while replacing five C-terminal FliF residues with Ile (see Fig. S1 for details). (a) Chemotaxis defect of the strain in a soft-agar migration assay. A tryptone soft-agar plate was spotted with 3 μl of overnight cultures and incubated for 6 h at 32 °C. Under the microscope, cells displayed vigorous tumbling. (b) Anti-FliG immunoblots of cell fractions, and whole cells, showing that native-sized FliG is not present at a significant level. w.c., whole cell; mem, membrane; cyto, cytosol; w.c. + FliG, whole-cell proteins from the fusion strain with native-sized FliG expressed from a plasmid (pDB97, with no induction). (c) Improved motility of the original FliF–FliG fusion strain when FliG or certain FliG variants are expressed from plasmids. The control (cont) is the original fusion strain with nothing else expressed. Plasmids were derivatives of pKP619, induced with 200 μM IPTG. Plates were spotted with 3 μl of overnight cultures and incubated for 12 h at 32 °C. The strain expressing wild-type FliG from the plasmid migrates at ~20% of the wild-type rate. FliGN, FliG amino-terminal domain; FliGMC, FliG middle and C-terminal domains. The right-hand plate shows the effects of expressing point-mutant variants of FliG that have been shown previously to abolish the function of the full-length protein [34,35]. Rescue of the fusion strain by extra FliG is diminished by a mutation in the N-terminal domain (L13D) but not by mutations in the middle or C-terminal domains. (d) Examples of suppressors of the chemotaxis defect in the fusion strain. Strains retained the FliF–FliG fusion and had acquired the indicated mutations in FliG or FliM. The original, unsuppressed FliF–FliG fusion is also shown (labeled “cont”). Plates were incubated for 12 h at 32 °C. Suppressor mutations are listed in Table 1.
