-
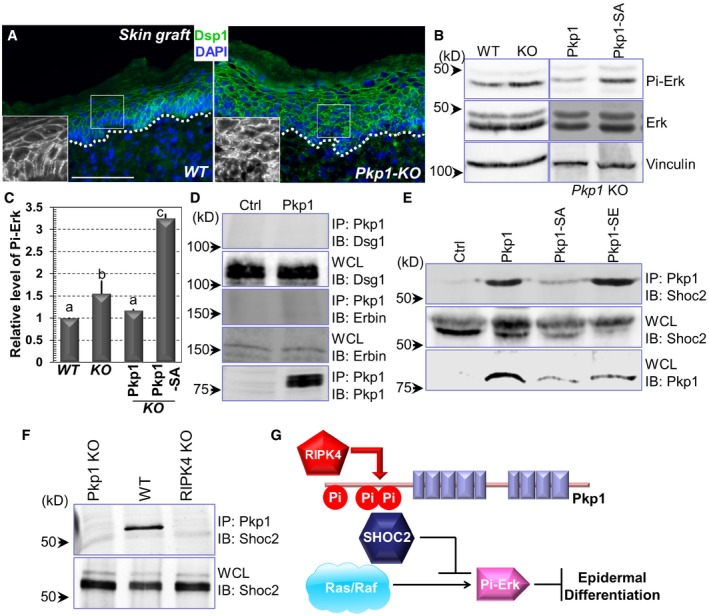
A
WT and Pkp1 KO skins were collected and subjected to immunofluorescence staining with antibody against Dsp1 (desmoplakin 1) as indicated. Scale bar = 50 μm.
-
B, C
Erk phosphorylation level was determined by Western blot analysis using WT, Pkp1 KO, and rescued cells. Band intensity was determined by densitometry and quantified in (C). Error bars represent SD. Data with different superscript letters are significantly different, P < 0.05 (one‐way ANOVA), n > 3.
-
D, E
Cell lysates were collected from different cell lines as indicated and immuno‐precipitated with α‐Pkp1 antibody. Immunoprecipitates (IP) and aliquots of whole‐cell lysate (WCL) were immunoblotted with different antibodies as indicated.
-
F
Cell lysates were collected from Pkp1 KO, WT Pkp1, and RIPK4 KO cells. Lysates were immuno‐precipitated with Pkp1 antibody and immunoblotted with different antibodies as indicated.
-
G
A working model of the regulation of epidermal differentiation by RIPK4 and Pkp1. Phosphorylation of Pkp1 by RIPK4 can suppress ERK signaling by association with SHOC2, which in turn enhances epidermal differentiation.