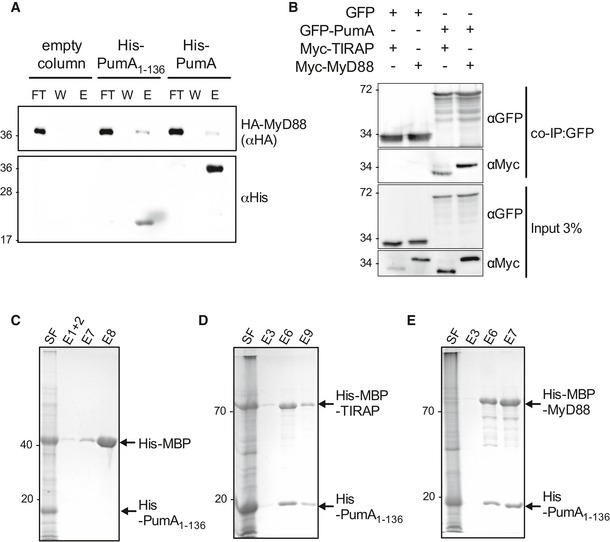
Figure 5. PumA is also capable of interacting with MyD88.
-
APull‐down assay using extracts from cells expressing HA‐MyD88 against His‐PumA or His‐PumA1–136 immobilized on a Ni‐NTA resin. Empty column was used as a control for non‐specific binding. Interactions were visualized by Western blotting using anti‐HA antibody, and column binding with anti‐His (lower blot). Non‐bound fraction (FT), last wash (W) and elution (E) are shown for each sample and the molecular weights indicated (kDa).
-
BCo‐immunoprecipitation (co‐IP) assay from cells expressing GFP‐PumA and either Myc‐TIRAP or Myc‐MyD88. GFP was used as a control for non‐specific binding. The co‐IP was revealed using an anti‐Myc antibody, the fraction bound to GFP‐trapping beads using an anti‐GFP antibody and the inputs (shown on the bottom two images) using both anti‐Myc and anti‐GFP antibodies.
-
C–ECo‐purification of His‐PumA1–136 co‐expressed in E. coli BL21 with either (C) His‐MBP (control), (D) His‐MBP‐TIRAP or (E) His‐MBP‐MyD88. Interactions were visualized with coomassie blue stained gels. Soluble fraction (SF) and selected elutions (E) are shown for each sample and the molecular weights indicated (kDa).
Source data are available online for this figure.
