Figure 3. Rhythmic clock gene expression emerges in (matured) cardiac cultures.
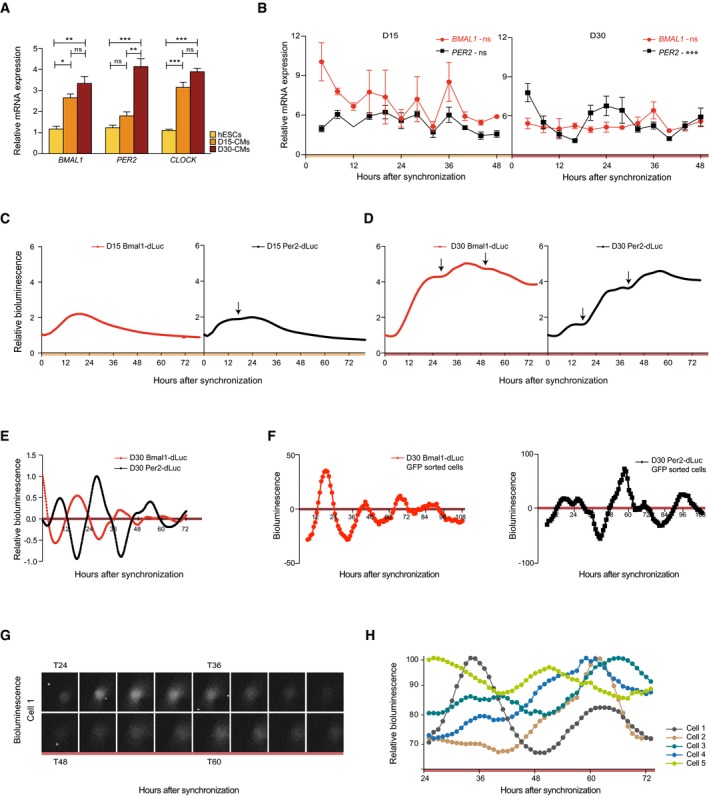
- BMAL1, PER2, and CLOCK expression levels at D0, D15, and D30 during directed cardiac differentiation as determined using qRT–PCR. Data are represented as mean ± s.e.m. of three independent replicates. Significant expression differences were tested by one‐way ANOVA, followed by a Bonferroni post hoc test (ns: not significant, *P < 0.05, **P < 0.005, ***P < 0.0005).
- qRT–PCR analysis of BMAL1 and PER2 expression over 48 h at a 4‐h interval in cardiac cells at D15. Expression levels in (A) and (B) were normalized to PPIA. Data are represented as mean ± s.e.m. of three independent replicates. Significance of rhythmicity across 48 h was analyzed using the RAIN algorithm and is indicated (ns: not significant, ***P < 0.0005).
- Promoter‐based destabilized luciferase (dLuc) reporter assay of the Bmal1 and Per2 promoter in synchronized cardiac cells at D15. Values are relative to T0. Measurements were performed using a LumiCycle32.
- Similar analysis as in (C) for D30.
- Detrended Bmal1‐dLuc and Per2‐dLuc luciferase signal measured in (D).
- Detrended Bmal1‐dLuc and Per2‐dLuc bioluminescence in NKX2‐5‐eGFP+ sorted and synchronized human ES cell‐derived cardiomyocytes at D30.
- Single‐cell analysis of Per2‐dLuc bioluminescence in sorted eGFP‐positive and synchronized D30 human ES cell‐derived cardiomyocytes.
- Representative Per2‐dLuc signal in five single D30 human ES cell‐derived cardiomyocytes over the course of 48 h.
