-
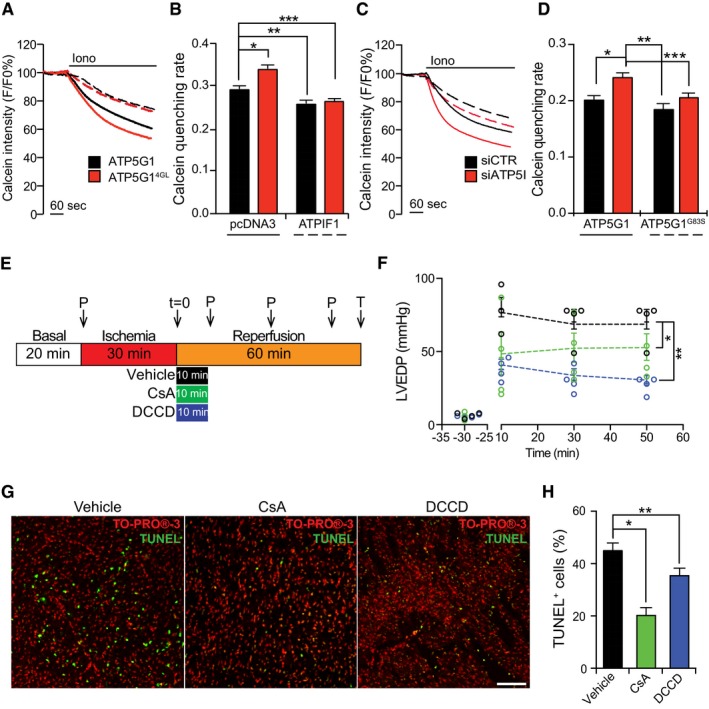
A, B
Representative calcein/Co2+ quenching recordings (A) and quenching rate quantification (B) in HEK293T cells transfected with an empty plasmid or with a construct for the overexpression of ATPIF1, combined with an ATP5G1‐ or an ATP5G14GL‐encoding plasmid, and exposed to 1 μM ionomycin (Iono). Results are representative of three independent experiments. *P = 0.0030, **P = 0.0035, ***P = 0.0078 (ANOVA plus unpaired Student's t‐test).
-
C, D
Representative calcein/Co2+ quenching recordings (C) and quenching rate quantification (D) in HEK293T cells transfected with a control siRNA (siCTR) or with a siRNA specific for ATP5I (siATP5I), combined with an ATP5G1‐ or an ATP5G1G83S‐encoding plasmid, and then treated with 1 μM Iono. Results are representative of three independent experiments. *P = 0.0001, **P = 0.00001, ***P = 0.0235 (ANOVA plus unpaired Student's t‐test).
-
E
Design of the experimental approach to study cardiac ischemia/reperfusion damage ex vixo. P, pressure readings. T, TUNEL assay.
-
F
Quantification of LVEDP in rat hearts subjected to ischemia ex vivo and then reperfused with vehicle only (black), or with vehicle supplemented with 0.2 μM cyclosporine A (CsA; green) or 0.2 μM N,N‐dicyclohexylcarbodiimide (DCCD; blue), as detailed in panel (E). Results are representative of four independent experiments and expressed as individual readings. *P = 0.0001, **P = 0.0032 (ANOVA plus unpaired Student's t‐test).
-
G, H
Representative images (G) and quantification (H) of TUNEL assays in rat hearts subjected to ischemia ex vivo and then reperfused with vehicle only, or with vehicle supplemented with 0.2 μM CsA or 0.2 μM DCCD, as detailed in panel (E). TO‐PRO®‐3 was employed as a nuclear counterstain. Results are representative of three independent experiments. *P = 0.0054, **P = 0.0006 (ANOVA plus unpaired Student's t‐test).
Data information: All results are expressed as mean ± SEM. Scale bar = 100 μm.