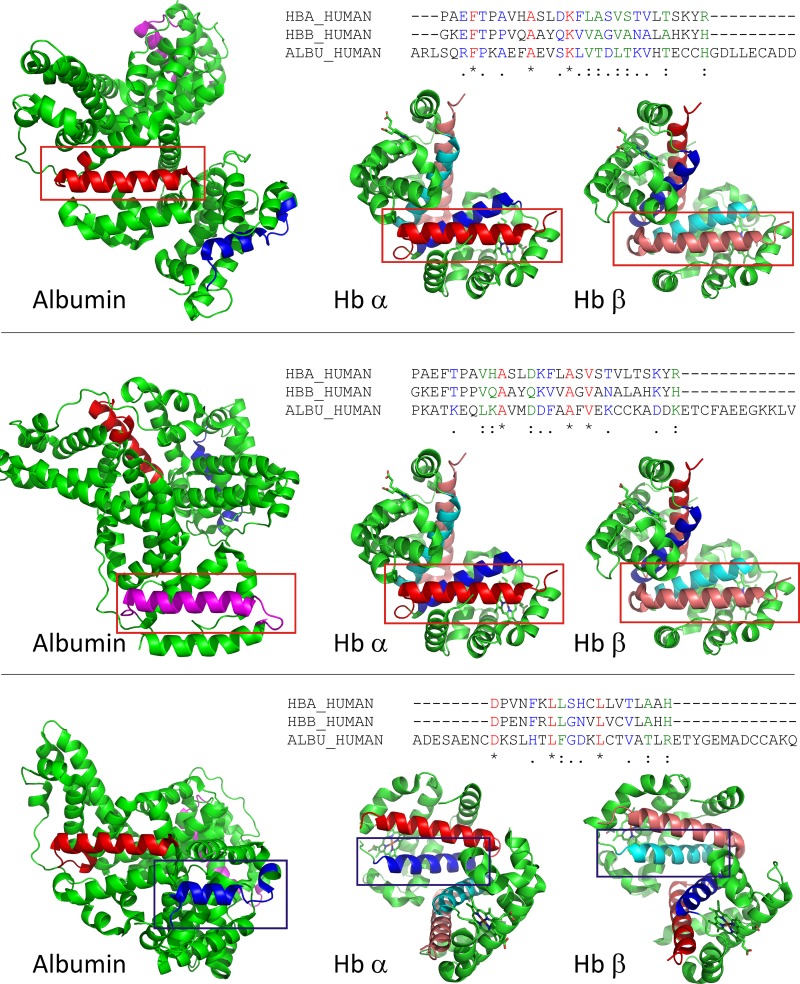
Fig. 6.
Sequence and structure comparison of potential megalin/cubilin binding regions in hemoglobin and albumin. Hb helical fragments involved in haptoglobin binding were aligned to human albumin sequence to search for comparable albumin motifs (see materials and methods for details). Three sequences in albumin were identified that have sequence and structural similarity to Hb. Top: alignment of Hb α- and β-helix H to albumin residues 244–271. The sequence alignment is shown on top. Aligned sequences are shown in the structures of albumin and Hb αβ-dimer and indicated by a red box. Helical elements are shown in red (albumin, hemoglobin α) and salmon (hemoglobin β); Middle: alignment of Hb α- and β-helix H to albumin residues 561–588. The sequence alignment is shown on top. Aligned sequences are shown in the structures of albumin and Hb αβ-dimer and indicated by a red box. Helical elements are shown in pink (albumin) red (hemoglobin α), and salmon (hemoglobin β) Bottom: alignment of Hb α- and β-helix G to albumin residues 87–105. The sequence alignment is shown on top. Aligned sequences are shown in the structures of albumin and Hb αβ-dimer and indicated by a blue box. Helical elements are shown in dark blue (albumin, hemoglobin α) and cyan (hemoglobin β).