Abstract
Background:
Spontaneous coronary artery dissection (SCAD) is a nonatherosclerotic but a rare and extremely dangerous clinical entity, it has a high prevalence in young female population with acute myocardial infarction (AMI). The previous reports were restricted to other countries’ population, but rare in China. Hence, this study aimed to focus on the characteristics of SCAD as a cause of young female AMI population in Jiangsu, China.
Methods:
This study enrolled young female AMI patients aged ≤50 years who underwent coronary angiography (CAG) and intracoronary imaging in our center between January 2013 and December 2016. Their clinical presentations, risk factors, and CAG characteristics were analyzed.
Results:
A total of 60 young female AMI (<7 days) patients were enrolled. From their CAG and intracoronary imaging results, the prevalence of SCAD in young female AMI population was 35% (21/60), the prevalence of coronary atherosclerostic heart disease was 65% (39/60). In the SCAD group, 43% (9/21) presented with non-ST-elevation myocardial infarction (NSTEMI) and the remainder presenting as STEMI. SCAD usually occurred in a single vessel (20/21, 95%), especially in left anterior descending artery (14/21, 67%). Eighteen patients (18/21, 86%) underwent conservative treatment, whereas the remaining three patients (3/21, 14%) underwent percutaneous coronary intervention. Regarding the angiographic results of SCAD lesions, intramural hematoma was discriminated in 95% (20/21), and Type I imaging was observed in 5% (1/21), Type II was observed in 67% (14/21), and Type III was 29% (6/21). The average stenosis in the group was 76.9% ± 20.6%, and the mean lesion length was 36.6 ± 8.6 mm.
Conclusions:
SCAD has a high prevalence in young female AMI population in Jiangsu, China. Discriminating the cause of AMI in young female population is very important.
Keywords: Acute Myocardial Infarction, Spontaneous Coronary Artery Dissection, Young Female
Introduction
Spontaneous coronary artery dissection (SCAD) is a nonatherosclerotic but a rare and extremely dangerous clinical entity, with a prevalence ranging from 0.1% to 1.0% in most angiographic series.[1] SCAD is defined as a spontaneous separation of coronary artery wall without any other reasons, it involves intimal tearing and hematoma formation within the artery media, causing compression the true lumen, and therefore results in angina, acute myocardial infarction (AMI), syncope, sudden death, and other clinical presentations. It usually affects younger and apparently healthy female population and accounted for 0.4% of sudden cardiac death.[1] The first case of SCAD was reported by Pretty[2] in 1931, a 42-year-old woman who died unexpectedly due to coronary artery dissection. Since then, SCAD publications comprised the case report and small case series, and hence the knowledge of SCAD was limited before. Until now, about 1500 SCAD cases have been reported worldwide,[3,4,5,6,7,8,9,10,11,12,13] but almost half of the cases were reported in the recent 5 years.
The previous reports showed the prevalence of SCAD in young female population with acute coronary syndrome (ACS) was 10%, and up to 25% in AMI population.[14] Some clinical characteristics of SCAD were gradually elaborated in the recent several years:[3,4,5,6,7,8,9,10,11,12,13,14] high prevalence as a cause of AMI in young female population, especially in those rarely with traditional risk factors, its more frequent angiographic presentation of diffused lesion but smoothly arterial narrowing wall, and its higher risk of recurrence.[15]
However, previous data were restricted to other countries’ population, rare systematic studies were reported in China. This study enrolled a series of young female AMI patients in the recent 4 years in the Centre of Cardiology, Nanjing First Hospital (Jiangsu, China), and focused on the characteristics of SCAD as a cause of AMI.
Methods
Ethical approval
As a retrospective study and data analysis was performed anonymously, this study was exempt from the ethical approval and informed consent from patients.
Study population
Inclusion criteria: young female patients (≥18 and ≤50 years) of AMI (≤7 days) who underwent coronary angiography (CAG) and intracoronary imaging (intravascular ultrasound [IVUS] or optical coherence tomography [OCT]) in our center between January 2013 and December 2016.
AMI was defined as the following criteria: (1) typical symptom such as chest pain lasting for more than 20 min; (2) 12-lead electrocardiogram presenting of new left bundle branch block or ST-segment changes in more than 2 contiguous leads; (3) serum troponin I/T increased more than twice of the upper limit of normal.[16]
SCAD was defined as a spontaneous separation of the coronary vessel wall together with typical diagnostic features (intimal dissection or intramural hematoma) identified by CAG and intracoronary imaging with IVUS or OCT,[17] percutaneous coronary intervention (PCI)-related dissection, iatrogenic, trauma, and atherosclerotic changes were excluded. Intimal dissection was defined as the presence of multiple radiolucent lumens, with or without contrast staining. The intramural hematoma was identified by an abrupt vessel tapering in concordance with recently proposed classification.[17]
SCAD based on the CAG imaging was classified into three types:[17] Type I (evident arterial wall staining): multiple radiolucent lumens of the coronary arterial wall with contrast staining. Type II (diffused stenosis but smoothly narrowing wall): often occurred in the mid to distal segments of coronary arteries, has diffused (typically >20 mm) lesion but smoothly narrowing wall. Type III (mimic atherosclerosis): long lesion (11–20 mm), hazy or linear stenosis but the lack of atherosclerotic changes in other coronary arteries. Type II and III need IVUS or OCT test to be confirmed. The result should be with an agreement in at least two independent cardiologists experienced in the angiographic diagnosis of SCAD.
Variables
Clinical presentation, demographics, laboratory results and CAG characteristics, treatments were collected through medical records and angiographic reviews. Detailed angiographic features included lesion location, lesion length, percentage of lesion vessel stenosis, percentage of PCI, intimal dissection or intramural hematoma, CAG imaging types were also assessed.
Statistical analysis
Data were presented as mean ± standard deviation (SD) for continuous variables and as frequency (%) for categorical variables. Student's t-test was used for comparison of normally distributed continuous data, and the Fisher's exact test was used to compare categorical variables. Statistical analysis was performed with the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) and P < 0.05 was considered statistically significant.
Results
A total of 60 young female patients (≤50 years) with AMI (≤7 days) were enrolled. Every patient not only had CAG but also IVUS/OCT results. All the SCAD cases had OCT/IVUS results confirmed. We divided them into two groups: SCAD group (21 patients) and coronary atherosclerotic heart disease (CHD) group (the remaining 39 patients). The prevalence of SCAD was 35% (21/60) in the population, and CHD was 65% (39/60).
Baseline clinical characteristics between spontaneous coronary artery dissection and coronary atherosclerotic heart disease groups
Clinical characteristics which including hypertension, current smoking, oral contraceptives, menopause, during pregnancy and the postpartum period, depression and autoimmune disease history distributed similar between the two groups. The mean age was 44.4 ± 4.1 years in SCAD group, only one fibromuscular dysplasia (FMD) case concomitant with SCAD was confirmed in the study due to peripheral artery angiography. Nevertheless, the mean age was 46.4 ± 3.8 years in CHD group, most of them often have more than one risk factor such as diabetes history, high glucose, low-density lipoprotein-cholesterol, triglyceride, total cholesterol, and non-high density lipoprotein-cholesterol levels [Table 1].
Table 1.
Baseline clinical characteristics of young female patients with AMI in the two groups
| Characteristics | SCAD group (n = 21) | CHD group (n = 39) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 44.4 ± 4.1 | 46.4 ± 3.8 | 1.885* | 0.064 |
| Hypertension | 7 (33) | 20 (51) | Fisher | 0.277 |
| Diabetes | 0 | 8 (21) | Fisher | 0.042 |
| Current smoking | 1 (5) | 0 | Fisher | 0.350 |
| Oral conceptives | 0 | 0 | – | – |
| Menopause | 11 (52) | 21 (54) | Fisher | 1.000 |
| Pregnancy period | 0 | 0 | – | – |
| Postpartum period | 0 | 0 | – | – |
| Depression | 0 | 0 | – | – |
| Autoimmune disease | 0 | 0 | – | – |
| FMD | 1 (5) | 0 | Fisher | 0.350 |
| Hb (g/L) | 121.9 ± 14.4 | 125.1 ± 14.3 | 0.809* | 0.422 |
| ALT (U/L) | 29.6 ± 18.7 | 33.5 ± 17.9 | 0.797* | 0.428 |
| Cr (µmol/L) | 51.5 ± 11.1 | 49.1 ± 15.8 | −0.611* | 0.544 |
| Glucose (mmol/L) | 5.2 ± 1.2 | 6.9 ± 2.8 | 3.456* | 0.001 |
| TC (mmol/L) | 3.6 ± 0.9 | 4.6 ± 1.7 | 3.128* | 0.003 |
| TG (mmol/L) | 1.3 ± 0.7 | 2.1 ± 1.4 | 2.626* | 0.011 |
| LDL-C (mmol/L) | 2.0 ± 0.7 | 3.0 ± 1.3 | 3.863* | <0.001 |
| HDL-C (mmol/L) | 1.2 ± 0.2 | 1.0 ± 0.2 | −3.904* | <0.001 |
| Non-HDL-C (mmol/L)† | 2.4 ± 0.9 | 3.6 ± 1.6 | 3.823* | <0.001 |
Data are presented as n (%) or mean ± standard deviation. *t values. SCAD: Spontaneous coronary artery dissection; CHD: Coronary atherosclerotic heart disease; FMD: Fibromuscular dysplasia disease; Hb: Hemoglobin; Cr: Creatinine; TC: Total cholesterol; TG: Total triglyceride; LDL-C: Low-density lipoprotein-cholesterol; HDL-C: High-density lipoprotein-cholesterol; †non-HDL-C = TC – HDL; –: Not applicable; AMI: Acute myocardial infarction; ALT: Alanine aminotransferase.
Characteristics of spontaneous coronary artery dissection group
In the SCAD group, 43% (9/21) presented with non-ST-elevation myocardial infarction (NSTEMI) and the remainder presenting as ST-elevation myocardial infarction (STEMI). SCAD usually occurred in a single vessel (20/21, 95%), especially in left anterior descending (LAD) artery (14/21, 67%), subsequently left circumflex coronary artery (3, 14%), right coronary artery (4, 19%). Eighteen patients (18/21, 86%) underwent conservative treatment, whereas the remaining three patients (3/21, 14%) underwent PCI. Regarding angiographic result of SCAD lesions, intramural hematoma was discriminated in 95% (20/21), intimal tearing only occurred in one case. Moreover, Type I was observed in 5% (1/21), Type II was observed in 67% (14/21) and Type III was 29% (6/21). The average stenosis in the group was 76.9% ± 20.6%, and the mean lesion length was 36.6 ± 8.6 mm [Figures 1–5].
Figure 1.
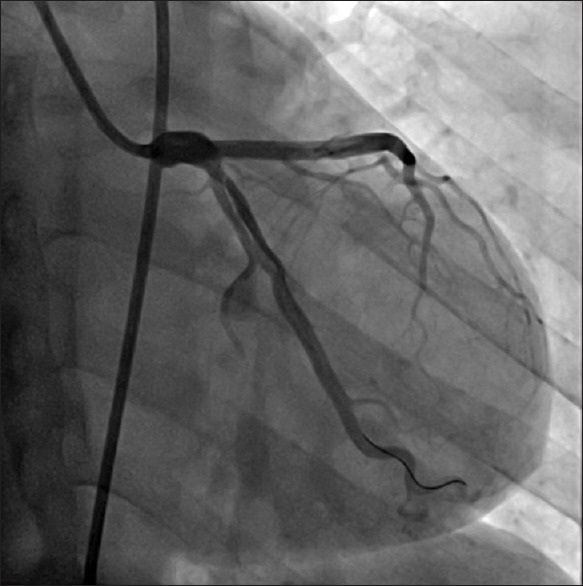
Type I: Multiple radiolucent lumens of the coronary arterial wall with contrast staining in the left anterior descending.
Figure 5.
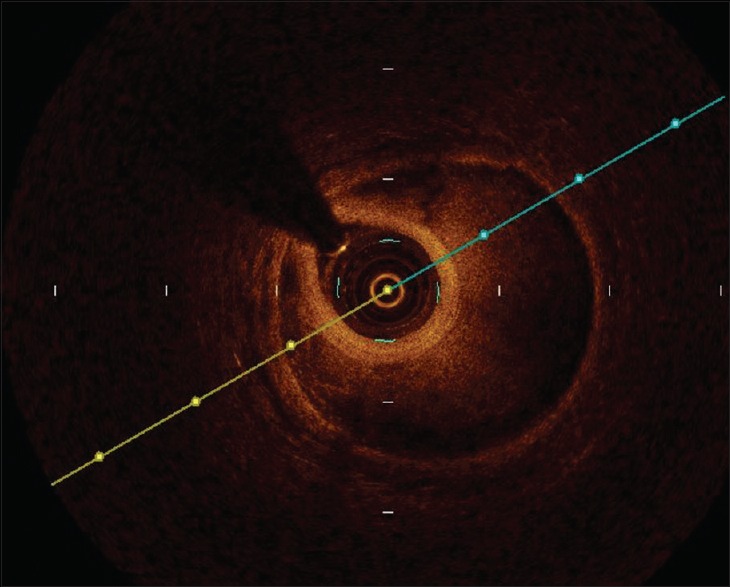
The true and false lumens from optical coherence tomography result which located at the frame from Figure 4.
Figure 2.
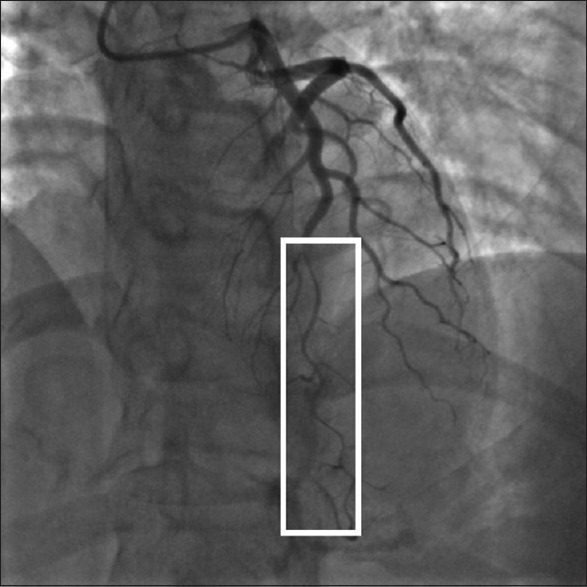
Type II: Diffused stenosis but smoothly narrowing in the mid to distal segment of left anterior descending.
Figure 3.
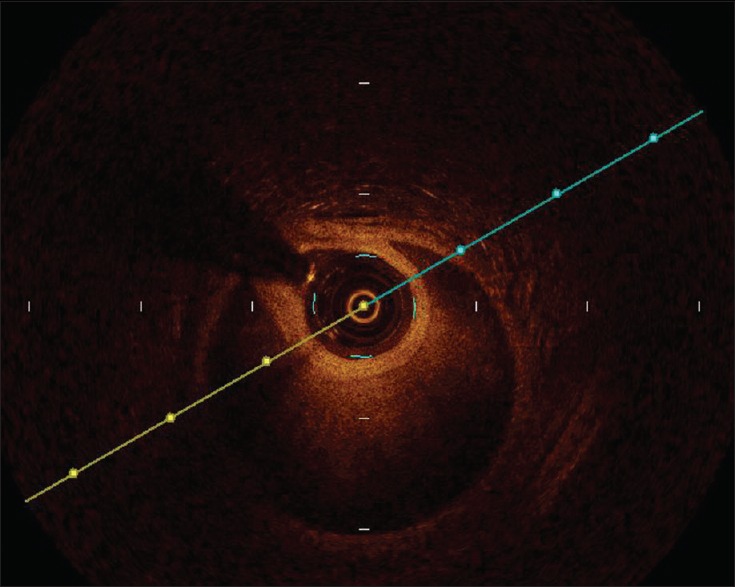
The true and false lumens from optical coherence tomography result which located at the frame from Figure 2.
Figure 4.
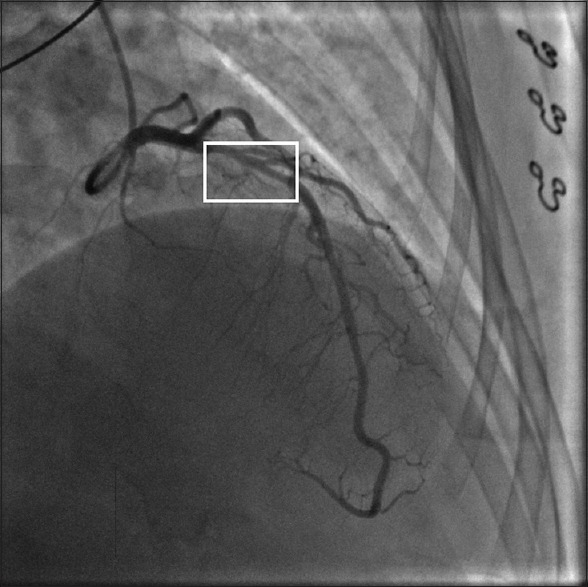
Type III: Mimic atherosclerosis, hazy or linear stenosis but lack of atherosclerotic changes in other coronary arteries.
Discussion
This study retrospectively reported a series of patients with SCAD as a cause of AMI in young female patients aged ≤50 years in China. Three main results can be taken from this study: (1) SCAD had a high prevalence in young female AMI population, especially with rare risk factors; (2) SCAD usually occurred in a single vessel, especially in LAD artery. (3) Most of SCAD cases were caused by intramural hematoma, and prone to had conservative drug treatment.
SCAD predominately occurred in younger women, with a 3-fold higher occurrence than in men,[18,19] but the reason of the difference remains poorly understood. A Japanese study showed that in a large young female AMI population (<55 years), the prevalence of CHD was 42%, SCAD was 35%, coronary artery spasm was 13%, and the others 10%.[13] However in the study, only two reasons were found (SCAD and CHD), might be due to our small sample and selection bias. A series of SCAD case reporting for the prevalence of SCAD in young female AMI patients was similar to that in our cohort:[11,13,15] a single-center Canadian study of young female AMI aged ≤50 years population from CAG examination, the prevalence of SCAD was 24.2%;[15] a Japanese series of young female AMI aged ≤50 years group, the prevalence of SCAD was 35%;[13] an Australian series of young female ACS aged ≤60 years group, the prevalence of SCAD was 22.5%.[11] Therefore, SCAD also had a high prevalence in young female AMI population in Jiangsu, China, it is probably the second cause of AMI in young female population regardless of ethnic and regional differences.
SCAD remains a distinct but challenging clinical entity, the risk factors of SCAD have poorly elaborated, may be associated with these factors:[1,14,20,21,22,23] (1) pregnancy and postpartum are high-risk periods in young women. The changed hormonal, hemodynamic, and thromboplastic factors level in this period might be predisposed to SCAD. (2) Intensive exercise or emotional depression is also considered of risk factor for SCAD. (3) Many systemic connective tissue disorders (Marfan's syndrome, Ehlers-Danlos, Loeys-Dietz syndromes, and systemic lupus erythematosus), autoimmune disease (rheumatoid arthritis, polyarteritis nodosa, Crohn's disease) have all been associated with SCAD. (4) FMD is another important disease concomitant with SCAD,[24,25,26,27,28,29] the prevalence of FMD ranged from 52% to 86% in SCAD patients.[4,7] FMD is a nonatherosclerotic and noninflammatory vascular disease, it tends to affect relatively large vessels such as renal, carotid artery, it can weaken artery structure through dysplasia of smooth muscle cells, fibroblasts, connective matrix, and prone to cause of artery stenosis, dissection, and aneurysm forming.[30] However, in this study, there were no cases in the period of pregnancy or postpartum, no emotional or intensive exercise precipitance records and none of them had a history of connective tissue or autoimmune disease. We only confirmed one FMD case, its prevalence was not as high as that described before, mainly due to our retrospective design of incomplete screening and unawareness of FMD before, so the prevalence of FMD concomitant with SCAD was probably underestimated than the truth in the study.
Saw et al.[7,8,9,10,11,13] reported that SCAD lesion mainly occurred at LAD artery and its branches (about 45–61%), subsequently circumflex and branches (15–45%), right coronary artery and branches (10% to 39%), and the left main stem (0 to 4%). The result showed the occurrence in LAD artery is a little higher than described before, the probable explanation might be the ethnic difference between Chinese and Western population and the small sample enrolled. On the other hand, the pathology of SCAD apparently has two mechanisms:[1,14,20,21,22,23] (1) a primary intimal rupture generates an entry door precipitating bleeding into the coronary wall with the development of a false lumen (“inside-out” mechanism). (2) A primary disruption of the “vasa vasorum” leading to intramural hemorrhage (“outside-in” mechanism). Higher prevalence of intramural hematoma was found in this study from OCT/IVUS result. Therefore, the data provide robust evidence that intramural hematoma might be the main pathology cause of SCAD in young female population.
In this study, the distribution of NSTEMI presentation was similar to STEMI in SCAD group, and most of them had conservative drug treatment. The distribution of clinical diagnosis was consistent with previous results.[11,13] The American Heart Association guidelines for AMI suggest early intervention for culprit lesion of the atherosclerotic vessel is beneficial, is it appropriate to SCAD population? The current guidelines do not give an optimal management of SCAD, the treatment of SCAD was still empirical.[4,5,6,7,8,31,32] Revascularization (containing PCI and coronary artery bypass grafting) is only recommended to those ongoing or recurrent ischemia patients, because it can quickly restore coronary flow to reduce infarct size, but stent implantation might lead to propagation of intramural hematoma and has a high risk of late stent malapposition, because the extent of intramural hematoma is often underappreciated through angiography, subsequently result in unanticipated loss of flow after stent placement and stent selected undersized, which can result to high risk of late stent malapposition. Conservative drug treatment might be helpful,[4,8,31,32] because SCAD tends to be spontaneous healing at follow-up. Patients who survived their initial SCAD presentation were often preferable at follow-up, because spontaneous vessel healing occurred in most patients. However, the recurrent rate was also higher compared with CHD patients, the recurrent SCAD occurring was about 15% at 2-year follow-up,[7] about 27% at 5-year follow-up.[8,13] No patient of the study died. However unfortunately, we did not have any follow-up data discharged.
The limitations included: first, the study was a retrospective, observational study of a relatively small population in a restricted district, the result can therefore only be considered exploratory. All the patients enrolled in the study must have complete CAG and OCT/IVUS imaging results, if the above data were incomplete, the patient would be excluded. Hence, the study had selection bias, the prevalence of SCAD in young female AMI population was probably underestimated than the truth in the study. Second, medical records were not explicit, so many risk factors remained insufficient for statistical analysis. Third, not every probable case had OCT/IVUS imaging data to be confirmed, and hence it also might underestimate the prevalence of SCAD. Forth, we did not have any follow-up data to observe their prognosis. Thus, these findings strongly suggest the need for a further, larger, randomized registry study to validate the above conclusions. We enrolled the population aged <50s, the next study will enlarge the age group containing <60s and compare the difference between them.
In conclusion, SCAD has a high prevalence in younger female AMI population in Jiangsu, China, especially in those rarely with traditional risk factors. Discriminating the cause of AMI in young female population is very important.
Financial support and sponsorship
This study was supported by grants from Nanjing Medical Science and Technique Development Foundation (No. YKK13108), and Nanjing Science and Technique Development Project (No. 2015sc511008).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Vrints CJ. Spontaneous coronary artery dissection. Heart. 2010;96:801–8. doi: 10.1136/hrt.2008.162073. doi: 10.1136/hrt.2008.162073. [DOI] [PubMed] [Google Scholar]
- 2.Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42: Rupture. BMJ. 1931;1:667. [Google Scholar]
- 3.Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: A case series. Circ Cardiovasc Interv. 2012;5:134–7. doi: 10.1161/CIRCINTERVENTIONS.111.966630. doi: 10.1161/CIRCINTERVENTIONS.111.966630. [DOI] [PubMed] [Google Scholar]
- 4.Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: Prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6:44–52. doi: 10.1016/j.jcin.2012.08.017. doi: 10.1016/j.jcin.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–88. doi: 10.1161/CIRCULATIONAHA.112.105718. doi: 10.1161/CIRCULATIONAHA.112.1058718. [DOI] [PubMed] [Google Scholar]
- 6.Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jiménez-Quevedo P, et al. Spontaneous coronary artery dissection: Long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. 2012;5:1062–70. doi: 10.1016/j.jcin.2012.06.014. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: Association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–55. doi: 10.1161/CIRCINTERVENTIONS.114.001760. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 8.Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: Revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–86. doi: 10.1161/CIRCINTERVENTIONS.114.001659. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 9.Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116:66–73. doi: 10.1016/j.amjcard.2015.03.039. doi: 10.1016/j.amjcard.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, et al. Spontaneous coronary artery dissection: Angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv. 2017;89:59–68. doi: 10.1002/ccd.26383. doi: 10.1002/ccd.26383. [DOI] [PubMed] [Google Scholar]
- 11.Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, Gulati R, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome – A single-centre Australian experience. Int J Cardiol. 2016;202:336–8. doi: 10.1016/j.ijcard.2015.09.072. doi: 10.1016/j.ijcard.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 12.Roura G, Ariza-Solé A, Rodriguez-Caballero IF, Gomez-Lara J, Ferreiro JL, Romaguera R, et al. Noninvasive follow-up of patients with spontaneous coronary artery dissection with CT angiography. JACC Cardiovasc Imaging. 2016;9:896–7. doi: 10.1016/j.jcmg.2015.06.011. doi: 10.1016/j.jcmg.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the angina pectoris-myocardial infarction multicenter investigators in Japan. Int J Cardiol. 2016;207:341–8. doi: 10.1016/j.ijcard.2016.01.188. doi: 10.1016/j.ijcard.2016.01.188. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso F. Spontaneous coronary artery dissection: New insights from the tip of the iceberg? Circulation. 2012;126:667–70. doi: 10.1161/CIRCULATIONAHA.112.122093. doi: 10.1161/CIRCULATIONAHA.112.122093. [DOI] [PubMed] [Google Scholar]
- 15.Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–9. doi: 10.1016/j.cjca.2014.01.011. doi: 10.1016/j.cjca.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Senter S, Francis GS. A new, precise definition of acute myocardial infarction. Cleve Clin J Med. 2009;76:159–66. doi: 10.3949/ccjm.75a.08092. doi: 10.3949/ccjm.75a.08092. [DOI] [PubMed] [Google Scholar]
- 17.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84:1115–22. doi: 10.1002/ccd.25293. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- 18.Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: Results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250–4. doi: 10.1016/j.ejcts.2008.10.023. doi: 10.1016/j.ejcts.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: A Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009;74:710–7. doi: 10.1002/ccd.22115. doi: 10.1002/ccd.22115. [DOI] [PubMed] [Google Scholar]
- 20.Alfonso F, Bastante T, Rivero F, Cuesta J, Benedicto A, Saw J, et al. Spontaneous coronary artery dissection. Circ J. 2014;78:2099–110. doi: 10.1253/circj.cj-14-0773. doi: 10.1253/circj.CJ-14-0773. [DOI] [PubMed] [Google Scholar]
- 21.Saw J. Spontaneous coronary artery dissection. Can J Cardiol. 2013;29:1027–33. doi: 10.1016/j.cjca.2012.12.018. doi: 10.1016/j.cjca.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Alfonso F, Bastante T. Spontaneous coronary artery dissection: Novel diagnostic insights from large series of patients. Circ Cardiovasc Interv. 2014;7:638–41. doi: 10.1161/CIRCINTERVENTIONS.114.001984. doi: 10.1161/CIRCINTERVENTIONS.114.001984. [DOI] [PubMed] [Google Scholar]
- 23.Giacoppo D, Capodanno D, Dangas G, Tamburino C. Spontaneous coronary artery dissection. Int J Cardiol. 2014;175:8–20. doi: 10.1016/j.ijcard.2014.04.178. doi: 10.1016/j.ijcard.2014.04.178. [DOI] [PubMed] [Google Scholar]
- 24.Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862–71. doi: 10.1056/NEJMra032393. doi: 10.1056/NEJMra032393. [DOI] [PubMed] [Google Scholar]
- 25.Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, et al. The United States registry for fibromuscular dysplasia: Results in the first 447 patients. Circulation. 2012;125:3182–90. doi: 10.1161/CIRCULATIONAHA.112.091223. doi: 10.1161/CIRCULATIONAHA.112.091223. [DOI] [PubMed] [Google Scholar]
- 26.Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, et al. Fibromuscular dysplasia: State of the science and critical unanswered questions: A scientific statement from the American Heart Association. Circulation. 2014;129:1048–78. doi: 10.1161/01.cir.0000442577.96802.8c. doi: 10.1161/01.cir.0000442577.96802.8c. [DOI] [PubMed] [Google Scholar]
- 27.Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Hum Pathol. 1987;18:654–6. doi: 10.1016/s0046-8177(87)80368-4. [DOI] [PubMed] [Google Scholar]
- 28.Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart. 2010;96:1119–25. doi: 10.1136/hrt.2009.185157. doi: 10.1136/hrt.2009.185157. [DOI] [PubMed] [Google Scholar]
- 29.Michelis KC, Olin JW, Kadian-Dodov D, d’Escamard V, Kovacic JC. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol. 2014;64:1033–46. doi: 10.1016/j.jacc.2014.07.014. doi: 10.1016/j.jacc.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, et al. Fibromuscular dysplasia: State of the science and critical unanswered questions: A scientific statement from the American Heart Association. Circulation. 2014;129:1048–78. doi: 10.1161/01.cir.0000442577.96802.8c. doi: 10.1161/01.cir.0000442577.96802.8c. [DOI] [PubMed] [Google Scholar]
- 31.Tweet MS, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: A disease-specific, social networking community-initiated study. Mayo Clin Proc. 2011;86:845–50. doi: 10.4065/mcp.2011.0312. doi: 10.4065/mcp.2011.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: Angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7:656–62. doi: 10.1161/CIRCINTERVENTIONS.114.001676. doi: 10.1161/CIRCINTERVENTIONS.114.001676. [DOI] [PubMed] [Google Scholar]


