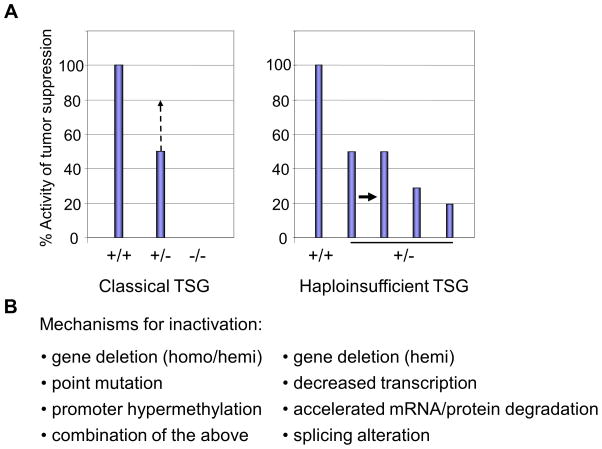
Figure 2. The activity of tumor suppressor genes/proteins in gene knockout mice (A) and possible mechanisms for inactivation of TSGs (B).
A. The wild type activity represents 100% diploid gene function and null (−/−) represents complete loss of function of the TSG. In classical TSGs, both loci are inactivated by i) point mutation, ii) gene deletion, iii) promoter hypermethylation, or iv) any combinations of these during tumor development. In this case, loss of one locus leads to compensatory activation of the remaining allele, leading to more than 50% of the tumor suppressor activity (left columns, middle, discontinued line; ref. 124). In haploinsufficient TSGs, tumors from mice with heterozygous gene deletion express the mRNA/protein at levels ~50% of wild type cells. The levels may be lower if other inactivation mechanisms co-exist to lower the levels of the tumor suppressive mRNA/protein (right columns). In p53+/− cells, both mRNA and protein levels are reduced to ~25% of those in wild type cells (48).
B. Possible mechanisms for inactivation of classical and haploinsufficient TSGs. Classical TSGs are inactivated by homozygous or heterozygous gene deletion plus point mutation or promoter hypermethylation(s). Conversely, haploinsufficient TSGs are inactivated by hemizygous gene deletion or point mutation at the single locus without promoter methylation or mutation of the remaining wild type allele. Recent studies show that other mechanisms, such as decreased transcription, accelerated RNA degradation (20), enhanced protein degradation, or aberrant splicing (72, 74, 119, 125–127) can coexist to down-regulate the TSG mRNA/protein expression to less than the 50% level of that in wild type cells in subsets of malignancies.