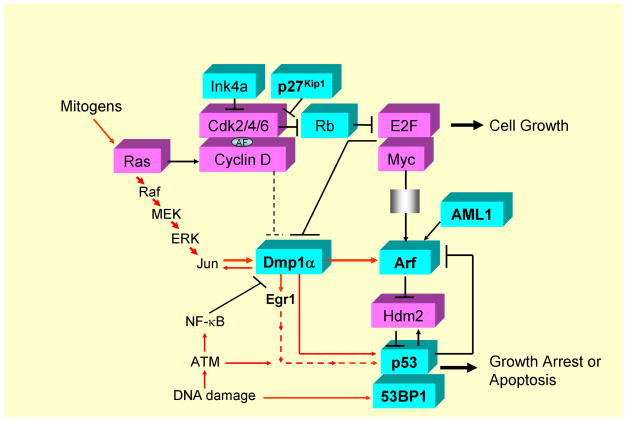
Figure 3. Intracellular signaling pathways involving p27Kip1, Dmp1, Arf, p53, 53BP1, and AML1.
Cyclin D/Cdks are activated in response to mitogenic signals and initiate phosphorylation of Rb, a process that is completed by cyclin E - Cdk2. Once cells enter the S phase, cyclin E is degraded and cyclin A enters into complexes with Cdk2. Proteins of the Ink4 family (p15, p16, p18, p19) bind only to Cdk4 and inhibit its activity while those in the Kip/Cip family (p21, p27, p57) inhibit cyclin-bound Cdks (25–28). Both p21Cip1 and p27Kip1 act as an assembly factor (AF; ref. 30). Arf is induced by potentially oncogenic signals stemming from overexpression of oncogenes such as c-Myc, E2F1, and activated Ras, which quenches inappropriate mitogenic signaling by diverting incipient cancer cells to undergo p53-dependent growth arrest or cell death (76, 77). Dmp1 is activated by oncogenic Ras or HER2, which, in turn, binds and activates the Arf promoter and induces cell cycle arrest (61, 65). Three different splicing variants have been reported for human DMP1 (72, 73). Dmp1 (Dmp1α) directly binds to the CD13 (128) and Arf (56) promoters to transactivate the gene expression, thereby accelerates myeloid cell differentiation by interfering cell cycle progression. Interestingly the CD13 promoter activation is inhibited by D-type cyclins in a Cdk-independent fashion (54, 128; see the dotted line for inhibition) while the Arf promoter activation is stimulated by cyclin D1 (129) in a Cdk-dependent fashion (57, 61). Other transcriptional targets for Dmp1α include Areg, Thbp-1, JunB, and Egr1 (118), suggesting that it is also involved in signal transduction related to angiogenesis and/or metastasis. Dmp1α physically interacts with p53 and neutralizes all the activities of Mdm2 to activate the p53 pathway (59, 60). Both Dmp1−/− and Dmp1+/− mice show hypersensitivity to develop tumors in response to carcinogen or γ-irradiation. E2F1-3a (130, 131) directly binds to the Dmp1 promoter and causes transcriptional repression (62). The Dmp1 promoter is repressed by NF-κB through direct binding of the promoter to RelA (63). Conversely, the Dmp1 promoter is activated by the oncogenic Ras-Raf-MEK-ERK-Jun pathway (61) and HER2-Pi3k-Akt-NF-κB (65), and thus Ras or HER2-driven carcinogenesis is dramatically accelerated in Dmp1-null mice. The transcription factor AML1 locus is frequently translocated to create hybrid molecules in human acute leukemias, and plays essential roles in normal hematopoiesis through dimerization of its partner CBFβ (91, 92). Direct transcriptional activation of the ARF promoter by AML1 and its inhibition by AML1-ETO has been reported (96). 53BP1 is a p53-binding protein, and is involved in DNA-damage response by choosing recombination and end joining at DNA double-strand breaks (50–52). Haploid insufficiency of p27Kip1, p53, 53BP1, Dmp1α, Arf, AML1, and Egr1 TSGs are discussed in this review. These genes are shown in bold. The DMP1 locus generates other two splice variants, namely DMP1β and γ, and the oncogenic role of DMP1β has recently been demonstrated in vivo (72–74). Aberrant splicing of TSGs and their roles in carcinogenesis are currently extensively studied (119, 125–127), which can further affect the function of the remaining allele of haploinsufficient TSGs. Proteins that have mitogenic/oncogenic functions are shown in dark while those with tumor-suppressive activities are shown in white.