Figure 3. Targeting RPTPσ with biologics.
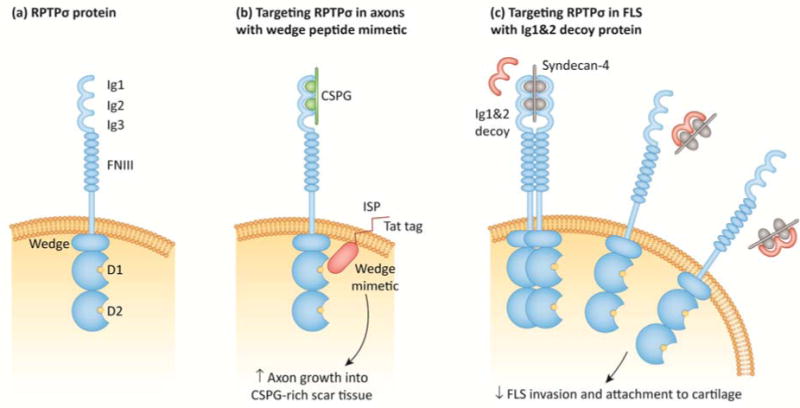
(a) Schematic of RPTPσ protein domains. RPTPσ consists of extracellular, transmembrane, and intracellular regions. The extracellular region consists of amino-terminal immunoglobulin-like domains (Ig1–Ig3) and multiple fibronectin type III (FNIII) repeats. As described in (b–c), the Ig1&2 domains mediate RPTPσ interactions with proteoglycans in the extracellular matrix (ECM). The intracellular region consists of a juxtamembrane helix-loop-helix “wedge” motif and two PTP domains (D1 and D2). (b) Targeting RPTPσ with a cell-penetrating wedge peptide mimetic (ISP). In neuronal cells, RPTPσ Ig1&2 domains act as a receptor for chondroitin sulfate proteoglycans (CSPG), an ECM component that is abundant in scar tissue and inhibits axon regeneration. Treatment with ISP, a wedge peptide mimetic conjugated to a cell-penetrating transactivator of transcription (TAT) peptide, alleviates CSPG-mediated inhibition of axon extension into CSPG-rich scars. The mechanism of action of ISP is currently unknown, although it likely inhibits RPTPσ function. (c) Targeting RPTPσ with an Ig1&2 decoy protein. On the surface of fibroblast-like synoviocytes (FLS), RPTPσ Ig1&2 domains bind to the heparan sulfate proteoglycan syndecan-4, which inhibits RPTPσ by inducing oligomerization. Treatment with recombinant Ig1&2 protein disrupts the interaction between RPTPσ and syndecan-4, inhibiting the invasiveness and cartilage attachment of FLS through RPTPσ catalytic activity.
