Figure 1. Key Figure. Optogenetics throughout the discovery pipeline.
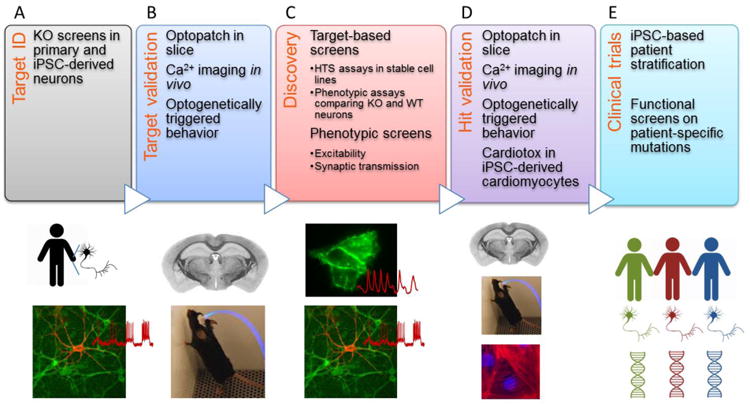
Optogenetic assays can contribute at each stage of drug discovery. A) Comparative measures on rodent or human neurons +/- a disease-causing mutation can establish a screenable phenotype. Alternatively, optogenetic screens of genetic knockouts can identify novel targets which can then be the subject of optogenetic or conventional drug screens. B) Targets identified in culture-based assays should be validated via knockout and functional measurements in acute slice, and ideally in vivo. Optogenetically triggered behaviors can provide a low-variance phenotype for testing the effects of mutations. C) Target-based optogenetic screens can be performed in a wide variety of cell-based assays. Alternatively, phenotypic optogenetic screens can identify compounds that modulate a disease-relevant functional phenotype regardless of mechanism. D) Screening hits should be validated in the same assays used for target validation. In addition, optogenetic assays in human iPSC-derived cardiomyocytes can help with cardiotoxicity assessment. E) Functional optogenetic measurements in human iPSC-derived neurons can seek to distinguish likely responders from non-responders prior to a clinical trial; or to match genetically heterogeneous patients with existing medications.
