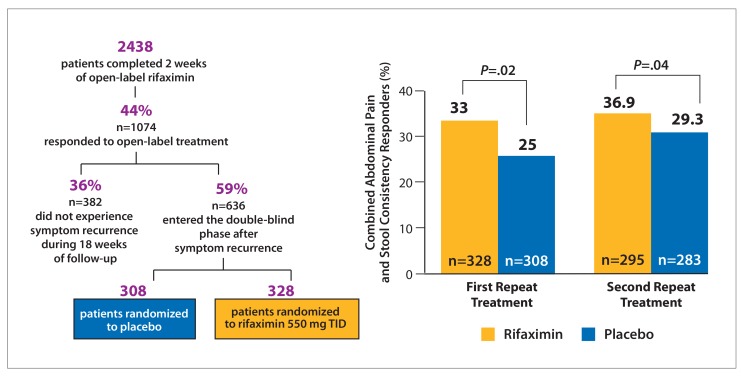
Figure 5.
Data from the TARGET 3 trial. Patient disposition (left)29 and proportion of composite abdominal pain and stool consistency responders (primary endpoint; right).28 Response was defined as ≥30% improvement baseline in the weekly average abdominal pain score and ≥50% reduction in the number of days per week with a daily stool consistency of Bristol Stool Form Scale type 6 or 7. TID, 3 times daily. Adapted from Lembo AJ et al. 2014 ACG Abstract 45,28 and Xifaxan [package insert]. Salix Pharmaceuticals: Bridgewater, NJ: 2015.29