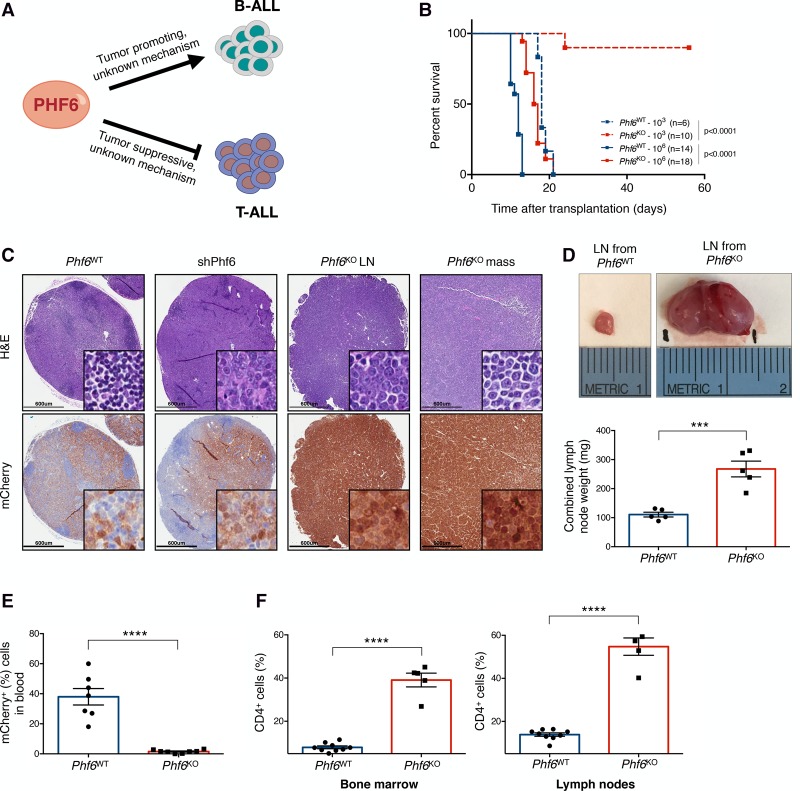
Figure 1.
Phf6 loss decreases the leukemogenic potential of cells in vivo and triggers a change in disease presentation. (A) PHF6 is a lineage-specific regulator of tumor growth in B-ALL and T-cell acute lymphoblastic leukemia (T-ALL). (B). Kaplan-Meier survival analysis of mice injected with either 103 (dotted) or 106 (solid) Phf6WT (blue) and Phf6KO (red) B-ALL cells. The number (n) of mice per genotype analyzed is shown. Statistical analysis (log-rank test, Mantel-Cox) was performed for the different groups in comparison with mice injected with Phf6WT cells. P-values are shown for the comparisons. (C) Representative hematoxylin and eosin (H&E) (top) and immunohistochemistry (bottom) staining of serial sections from lymph nodes (LNs) and lymphoma (mass) of recipient mice injected with Phf6WT, shPhf6, and Phf6KO cells. mCherry immunochemistry demarcates tumor cells. Bars, 600 µm. (D, top) Size comparison of representative LNs from Phf6WT (left) and Phf6KO (right) recipient mice. (Bottom) Quantification of combined LN weight of Phf6WT (blue; n = 5) and Phf6KO (red; n = 5) recipients. (E) Tumor burden in the blood of Phf6WT (blue; n = 7) and Phf6KO (red; n = 8) recipient mice. mCherry demarcates tumor cells. (F) Bar graphs showing the percentage of the CD4+ fraction among mCherry+ cells isolated from Phf6WT (blue; n = 9) and Phf6KO (red; n = 5) tumors in bone marrow (left) and LNs (right). Data represent the mean ± standard deviation (SD) in D–F. Statistics were calculated with two-sided Student's t-test. (***) P < 0.001; (****) P < 0.0001.