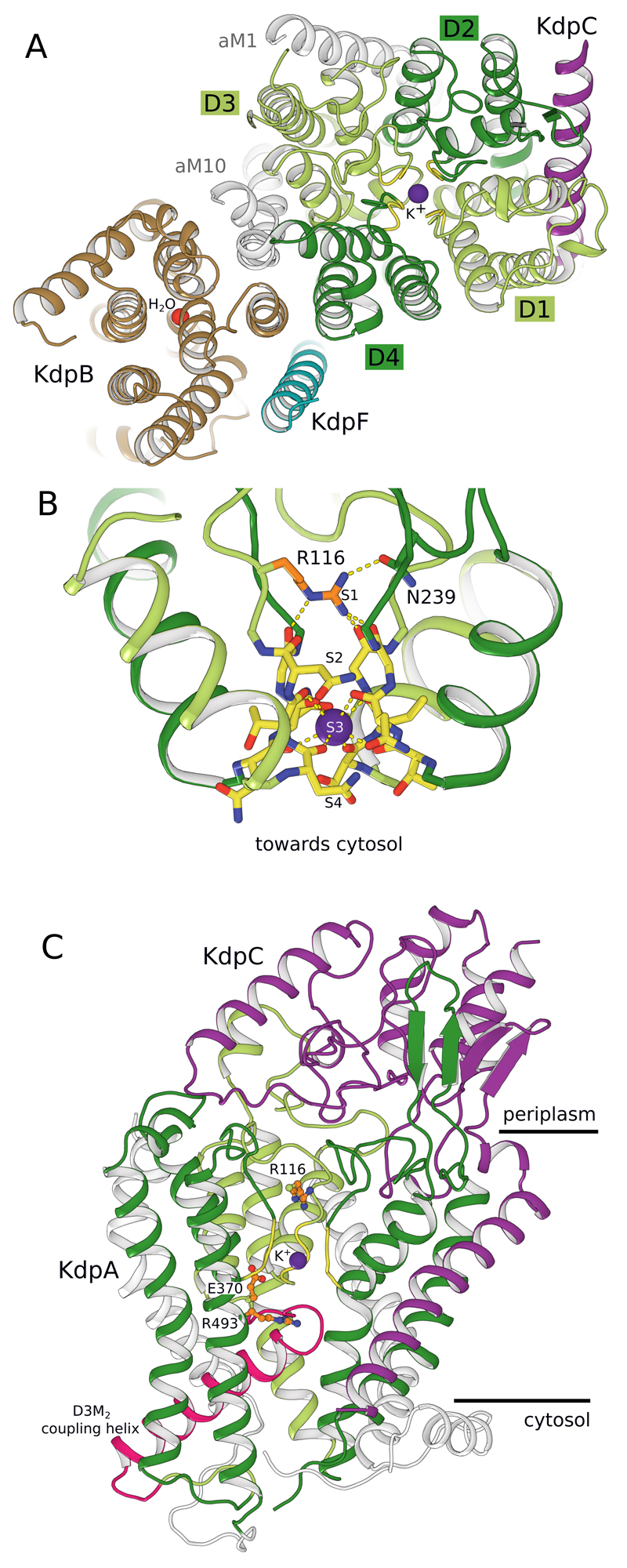
Figure 2. Potassium binding by KdpA.
(A) KdpA, as seen from the periplasmic side, has four M1PM2 units (D1-D4) that compose the selectivity filter (yellow backbone) and bind K+ (purple sphere). KdpB (brown) includes a water molecule (red sphere) at its canonical cation binding site. (B) Side view of the KdpA selectivity filter shows Arg116 in the S1 site and main chain carbonyls coordinating K+ in the S3 site. (C) Side view shows the gating loop below the selectivity filter with the coupling helix (pink). On the periplasmic side, the soluble domain of KdpC (purple) is firmly held by loops from repeat D2 (dark green) and D3 (light green) of KdpA (Extended Data Fig. 2).