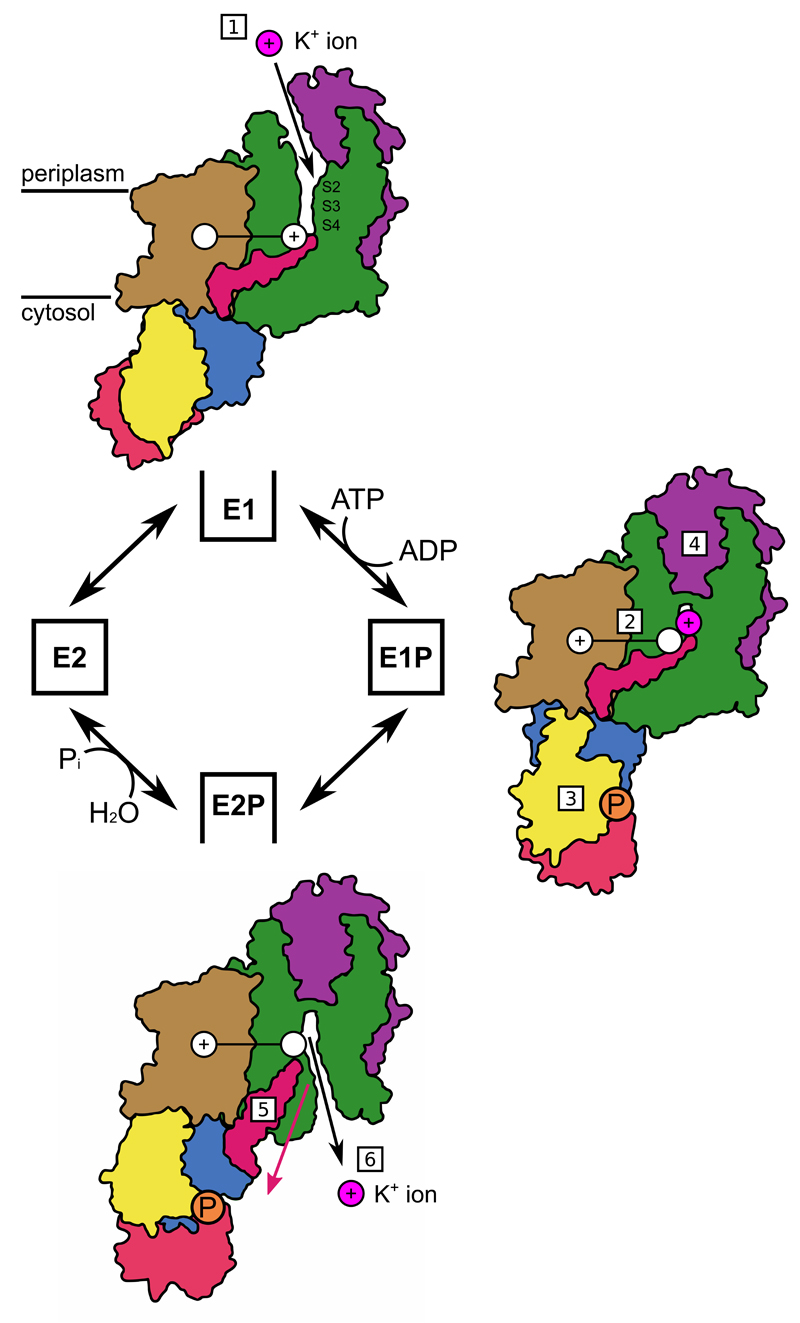
Figure 4. Mechanism for KdpFABC.
According to the Post-Alberts Scheme, transitions between open and closed states (black boxes) are driven by phosphorylation and dephosphorylation events in the cytosolic domains of KdpB. The cycle is initiated by K+ binding to the E1 state from the periplasm [1]. The presence of potassium in the S4 site of the selectivity filter of KdpA leads to charge transfer through the tunnel to the transmembrane domain of KdpB [2]. Presence of charge at the canonical site in KdpB triggers phosphorylation through a conserved P-type ATPase mechanism [3]. K+ occlusion, which may involve the periplasmic domain of KdpC, leads to the occluded E1P state [4]. The transition to the E2P state in P-type ATPases involves inclination of the P-domain away from KdpA, which will pull the D3 coupling helix (pink) of KdpA [5]. This movement opens the cytoplasmic gate, thus allowing K+ release to the cytosol [6]. The models are derived from our structure of an inhibited E1 state and SERCA1a structures of E1P (1T5T) and E2P (3B9B) states.